Vitamin D Supplementation in Older Adults: A 2025 Review of Clinical Guidelines, Research Gaps, and Translational Challenges
This article provides a comprehensive analysis of current vitamin D supplementation guidelines for older adults, tailored for researchers and drug development professionals.
Vitamin D Supplementation in Older Adults: A 2025 Review of Clinical Guidelines, Research Gaps, and Translational Challenges
Abstract
This article provides a comprehensive analysis of current vitamin D supplementation guidelines for older adults, tailored for researchers and drug development professionals. It synthesizes foundational evidence from the 2024 Endocrine Society Clinical Practice Guideline and other recent sources, explores methodological approaches for clinical application, addresses optimization challenges in real-world settings, and offers a critical validation of guideline consensus and disparities. The review identifies persistent evidence gaps and outlines future directions for biomedical research, including the need for standardized dosing, personalized protocols, and investigation into non-skeletal health outcomes.
The Evolving Scientific Basis for Vitamin D Supplementation in Aging Populations
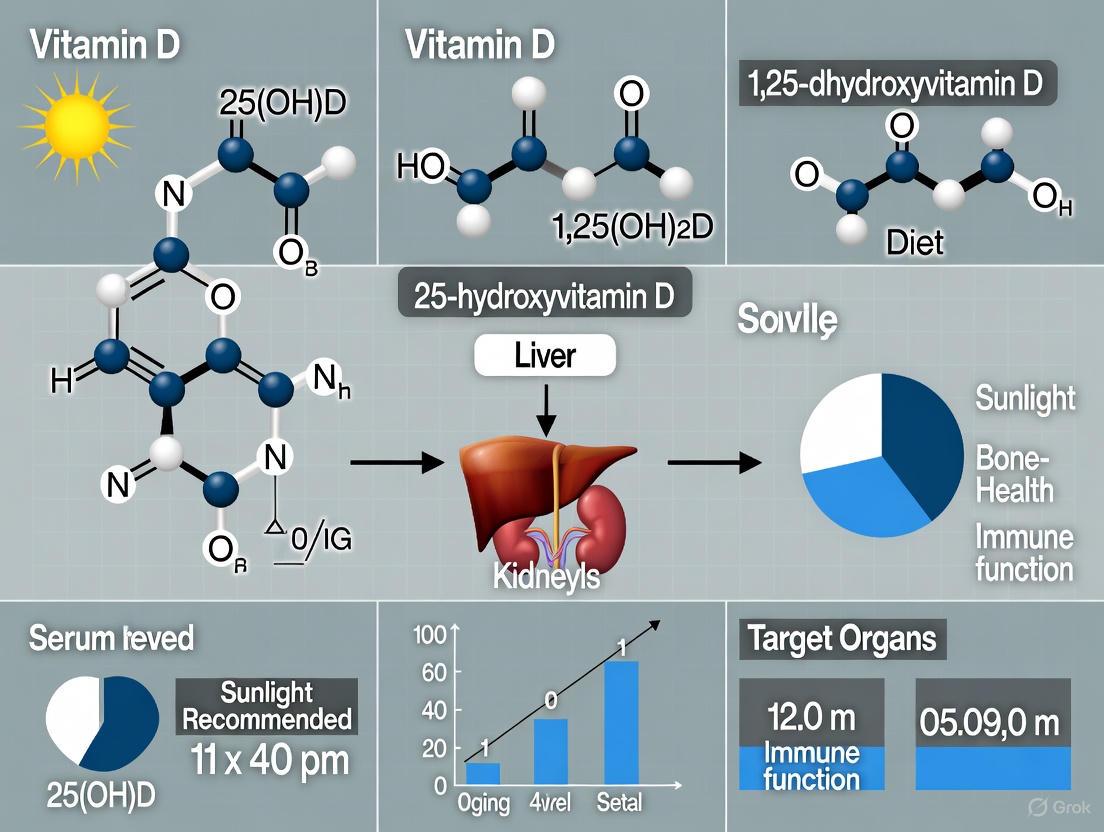
Vitamin D metabolism undergoes significant physiological shifts with advancing age, creating a heightened risk of deficiency in older adults. These changes impact the initial synthesis of vitamin D in the skin as well as its subsequent absorption and activation. This review, framed within the context of updating vitamin D supplementation guidelines for older adults, details the specific alterations in vitamin D physiology associated with aging. It provides a structured summary of quantitative data, delineates key experimental methodologies for investigating these shifts, and illustrates the critical molecular pathways involved, offering a resource for researchers and drug development professionals.
The table below summarizes the key quantitative changes in vitamin D physiology that occur with aging.
Table 1: Age-Related Physiological Shifts in Vitamin D Metabolism
| Physiological Process | Impact of Aging | Key Evidence/Mechanism |
|---|---|---|
| Cutaneous Synthesis | Substantial decline in the production of vitamin D₃ (cholecalciferol) upon exposure to UVB sunlight [1] [2]. | Reduced levels of its precursor, 7-dehydrocholesterol, in the skin [3]. |
| Intestinal Absorption | Reduced absorption efficiency of dietary vitamin D [1] [2]. | Impaired incorporation into mixed micelles and potential dysregulation of intestinal transport proteins (e.g., SR-B1, NPC1L1, CD36) [1]. |
| Serum 25(OH)D Level | High prevalence of deficiency and inadequacy in the older adult population [4]. | In one study, 41% of non-hospitalized patients aged 49 to 83 were deficient [4]. |
| Recommended Daily Allowance (RDA) | Higher requirement to compensate for reduced synthesis and absorption [2] [5]. | National Academy of Medicine RDA: 800 IU/day for adults ≥70 years vs. 600 IU/day for younger adults [5]. |
Experimental Protocols for Investigating Age-Related Changes
Protocol: Assessing In Vivo Wound Healing and Molecular Pathways in Aged Models
This protocol is adapted from studies investigating vitamin D's effect on wound healing in aged mice [3].
- Primary Objective: To evaluate the efficacy of vitamin D supplementation in accelerating wound closure in aged skin and to elucidate the underlying molecular mechanisms.
- Experimental Model:
- Animals: Aged (e.g., 12-month-old) female C57BL/6 J mice. A young (e.g., 3-month-old) group serves as a control for baseline age-related deficits [3].
- Supplementation: Administer vitamin D₃ (cholecalciferol) orally via drinking water or diet for a pre-treatment period (e.g., 3 months) prior to wounding and throughout the healing period [3].
- Control Groups: Include an aged control group receiving a vehicle and a young control group.
- Wound Creation:
- Anesthetize mice according to approved ethical guidelines.
- Create standardized, full-thickness excisional wounds on the dorsum using a biopsy punch.
- Outcome Measures:
- Wound Closure Kinetics: Measure wound area regularly using digital photography and planimetry software until complete closure.
- Tissue Collection: Harvest wound tissue and serum at predetermined timepoints post-wounding.
- Molecular Analysis:
- Histology & Immunohistochemistry: Assess re-epithelialization, angiogenesis (CD31 staining), and immune cell infiltration (e.g., F4/80 for macrophages).
- Gene Expression: Quantify mRNA levels of inflammatory cytokines (IL-6, TNF-α, IL-10), angiogenic factors (VEGF, VEGFR2), and epithelial-mesenchymal transition (EMT) markers via qRT-PCR.
- Protein Analysis: Measure levels of key proteins in the Hippo pathway (p-YAP, YAP, TAZ, Mst1, Lats1) and other targets via Western blot.
- Data Analysis: Compare the rate of wound closure and molecular marker expression between supplemented and control groups.
Protocol: Quantifying Telomere Length in Vitamin D Supplementation Trials
This protocol is based on large-scale randomized controlled trials like VITAL and DO-HEALTH that investigated vitamin D's effect on cellular aging [6] [7] [8].
- Primary Objective: To determine if vitamin D supplementation slows age-associated telomere shortening in human participants.
- Study Design:
- Type: Randomized, double-blind, placebo-controlled trial.
- Participants: Community-dwelling older adults (e.g., ≥50 or ≥70 years old) [7] [8].
- Intervention: Daily oral supplementation with a defined dose of vitamin D₃ (e.g., 2,000 IU) versus a matched placebo. Follow-up duration of several years (e.g., 3-5 years) is typical [6] [8].
- Sample Collection and Processing:
- Collect peripheral blood samples at baseline and at regular intervals during follow-up.
- Isolate leukocytes (white blood cells) or specific subpopulations from whole blood using density gradient centrifugation.
- Extract genomic DNA from the isolated cells using standard commercial kits.
- Telomere Length Measurement:
- Method: Quantitative Polymerase Chain Reaction (qPCR) is a common high-throughput method [5].
- Procedure: Amplify telomeric DNA sequences and a single-copy reference gene (e.g., 36B4) in parallel reactions. The telomere length is expressed as the ratio of telomere repeat copy number to the single-copy gene copy number (T/S ratio).
- Data Analysis: Use analysis of covariance (ANCOVA) to compare the change in telomere length from baseline to the end of the study between the vitamin D and placebo groups, adjusting for baseline telomere length and other covariates like age and BMI [8].
Signaling Pathways and Molecular Mechanisms
Vitamin D exerts its effects on aged skin through complex signaling pathways. The diagram below illustrates the key pathway by which vitamin D promotes wound healing via the Hippo pathway, based on experimental findings [3].
Diagram Title: Vitamin D Promotes Wound Healing via the Hippo Pathway
The Scientist's Toolkit: Research Reagent Solutions
The table below catalogs essential reagents and materials for conducting research on vitamin D and skin aging.
Table 2: Key Research Reagents for Vitamin D and Skin Aging Studies
| Reagent/Material | Function/Application | Examples/Specifications |
|---|---|---|
| Vitamin D Metabolites & Analogs | Used for in vitro and in vivo supplementation studies to activate VDR. | 1α,25(OH)₂D₃ (Calcitriol): Active form [3].Cholecalciferol (Vitamin D₃): Form for dietary/oral supplementation [3].Calcipotriol: Synthetic analog for topical application [3]. |
| Cell Lines | In vitro models for mechanistic studies on keratinocyte function, migration, and signaling. | HaCaT Cells: Immortalized human keratinocyte cell line [3].Primary Human Keratinocytes: Isolated from young and aged donors for age-comparative studies. |
| Antibodies for Immunoassay | Detection and quantification of key proteins in tissue sections (IHC) or cell lysates (Western blot). | Hippo Pathway: Anti-YAP/TAZ, anti-p-YAP, anti-Mst1, anti-Lats1 [3].Angiogenesis: Anti-CD31 (PECAM-1) [3].EMT Markers: Anti-E-cadherin, Anti-N-cadherin, Anti-Vimentin [3].Macrophage Polarization: Anti-iNOS (M1), Anti-CD206 (M2). |
| ELISA/Kits | Quantitative measurement of soluble factors in serum, plasma, or tissue homogenates. | 25-Hydroxyvitamin D (25(OH)D) EIA/ELISA: Gold standard for assessing vitamin D status [5].Cytokine Panels: For IL-6, TNF-α, IL-10, etc. [3]. |
| Pathway Inhibitors/Agonists | Tools for validating the specific role of a signaling pathway in the observed vitamin D effects. | Verteporfin: YAP inhibitor, used to block Hippo signaling downstream of vitamin D [3]. |
| qPCR Assays | Gene expression analysis of vitamin D targets, inflammatory markers, and pathway components. | TaqMan or SYBR Green assays for VDR, CYP27B1, CYP24A1, VEGF, and EMT transcription factors [3]. |
The definition of optimal serum 25-hydroxyvitamin D (25(OH)D) concentrations remains a significant challenge in clinical research and practice. Despite decades of investigation, consensus on precise thresholds for vitamin D sufficiency and deficiency has been elusive, with recommendations evolving as new evidence emerges. This ongoing refinement reflects the complexity of vitamin D metabolism and its pleiotropic effects across different physiological systems and population subgroups. The recent 2024 Endocrine Society Clinical Practice Guideline marked a pivotal shift by acknowledging that "in healthy adults, 25(OH)D levels that provide outcome-specific benefits have not been established in clinical trials" [9]. This statement underscores the current limitations in evidence and highlights the context-dependent nature of vitamin D threshold definitions.
The determination of optimal 25(OH)D concentrations is further complicated by varying definitions used by different professional organizations and research bodies. The Institute of Medicine (IOM) considers a minimal 25(OH)D concentration of 20 ng/mL (50 nmol/L) as physiologically adequate for at least 97.5% of the population [9], while the Endocrine Society in 2011 recommended serum levels of >30 ng/mL (>75 nmol/L) as optimal [9]. This lack of standardization presents challenges for researchers and clinicians in interpreting study results and applying guidelines consistently across patient populations. Furthermore, threshold requirements may vary based on specific health outcomes, age groups, and clinical conditions, necessitating a more nuanced approach to defining vitamin D status.
Current Guidelines and Threshold Recommendations
Comparative Analysis of Organizational Thresholds
Table 1: Comparison of 25(OH)D Threshold Definitions from Major Organizations
| Organization | Deficiency | Insufficiency | Sufficiency | Notes |
|---|---|---|---|---|
| Institute of Medicine (IOM) [9] | <12 ng/mL (<30 nmol/L) | 12-20 ng/mL (30-50 nmol/L) | ≥20 ng/mL (≥50 nmol/L) | Considered adequate for 97.5% of population |
| Endocrine Society (2011) [9] | <20 ng/mL (<50 nmol/L) | 21-29 ng/mL (51-74 nmol/L) | ≥30 ng/mL (≥75 nmol/L) | Updated 2024 guidelines do not specify reference values |
| Endocrine Society (2024) [10] [9] | Not specified | Not specified | Not specified | Acknowledges outcome-specific benefits not established in healthy adults |
| Common Research Definitions [11] | <20 ng/mL (<50 nmol/L) | 20-30 ng/mL (50-75 nmol/L) | >30 ng/mL (>75 nmol/L) | Frequently used in clinical studies |
| Severe Deficiency [11] | <12 ng/mL (<30 nmol/L) | - | - | Associated with rickets and osteomalacia |
Special Population Considerations
Threshold requirements vary significantly across different population subgroups, reflecting distinct physiological needs and risk profiles. For older adults, specifically those aged 75 years and above, the 2024 Endocrine Society guidelines recommend vitamin D supplementation higher than the IOM recommended daily allowance based on potential mortality risk reduction [12]. This population demonstrates increased vulnerability to vitamin D deficiency due to reduced skin synthesis and decreased intestinal absorption of vitamin D [13]. The Finnish fortification program has demonstrated that population-wide approaches can significantly improve vitamin D status, though interestingly, a recent study found that serum 25(OH)D sufficiency did not directly correlate with better muscle mass or function among middle-aged and older Finnish populations [14]. This suggests that threshold benefits may be outcome-specific rather than universally applicable.
For pediatric populations, threshold definitions remain particularly contentious. A 2025 study challenged the IOM's threshold of <12 ng/mL for infants, suggesting that the optimal inflection point for serum 25-OH-D was significantly lower at 6.83 ng/mL (95% CI, 5.02–8.64) when using parathyroid hormone (PTH) levels as a functional indicator [15]. This highlights the potential overestimation of vitamin D deficiency prevalence when applying adult-derived thresholds to pediatric populations. Research by Zhu et al. further demonstrated that applying different diagnostic criteria dramatically alters deficiency classification in children aged 0-6 years [16]. Using stricter criteria (Criterion II: deficiency <50 nmol/L), 12.43% were classified as deficient, compared to only 2.46% using more lenient criteria (Criterion I: deficiency <30 nmol/L) [16]. This classification discrepancy underscores the critical impact of threshold selection on epidemiological data and clinical decision-making.
Disease-Specific Optimal 25(OH)D Levels
Evidence from Dose-Response Meta-Analyses
Table 2: Disease-Specific Optimal 25(OH)D Concentrations Based on Meta-Analyses
| Health Outcome | Optimal 25(OH)D Level | Source/Study Type | Risk Reduction |
|---|---|---|---|
| Colorectal Cancer | ~34 ng/mL (85 nmol/L) | Gorham ED et al. 2007 [9] | 50% reduction in incidence |
| Breast Cancer | ~31 ng/mL (78 nmol/L) | Grant WB 2010 [9] | 50% reduction in incidence rate |
| Type 2 Diabetes | ~40 ng/mL (100 nmol/L) | Song Y et al. 2013 [9] | Significantly lower risk |
| Cardiovascular Disease | ~24 ng/mL (60 nmol/L) | Wang L et al. 2012 [9] | Higher risk below this level |
| All-Cause Mortality | ~28 ng/mL (70 nmol/L) | Schöttker B et al. 2014 [9] | Lowest mortality risk |
| Hip Fracture | ≥24 ng/mL (≥61 nmol/L) | Bischoff-Ferrari HA et al. 2012 [9] | Reduced fracture risk |
Dose-response meta-analyses have revealed that different health outcomes associate with different optimal 25(OH)D concentrations. The majority of disease-specific recommendations set a lower limit of 75 nmol/L and an upper limit of approximately 125 nmol/L for optimal 25(OH)D levels [9]. For cancer prevention, particularly breast and colorectal cancer, optimal levels appear higher, in the range of 75-110 nmol/L [9]. This pattern suggests that tissue-specific requirements for vitamin D may vary, potentially reflecting differences in vitamin D receptor expression or localized metabolism in different organ systems.
The shape of association between 25(OH)D levels and disease risk also differs across outcomes. Some conditions demonstrate linear relationships (e.g., type 2 diabetes), while others show threshold effects (e.g., fracture risk) or even U-shaped relationships (e.g., lung cancer) where both low and very high levels may associate with increased risk [9]. These diverse relationships complicate the establishment of universal optimal ranges and support the concept of outcome-specific target levels. For older adults, the relationship between vitamin D status and musculoskeletal health is particularly relevant, with evidence suggesting that levels ≥61 nmol/L are associated with reduced risk of hip and non-vertebral fractures [9].
Experimental Protocols for Threshold Determination
Protocol 1: Establishing Thresholds via PTH Inflection Point Analysis
Background: This method determines vitamin D deficiency based on the inverse relationship between 25(OH)D and parathyroid hormone (PTH), where rising PTH indicates inadequate vitamin D status for bone mineral homeostasis [15].
Materials and Reagents:
- Serum collection tubes
- 25(OH)D immunoassay kit
- Intact PTH immunoassay kit
- Statistical software (R, SPSS, or equivalent)
Procedure:
- Recruit study population representative of target demographic (e.g., infants, older adults)
- Collect fasting blood samples under standardized conditions
- Measure 25(OH)D and PTH levels using validated assays
- Plot PTH values against 25(OH)D concentrations
- Use piecewise linear regression or nonlinear models to identify the 25(OH)D level at which PTH begins to rise significantly
- Calculate inflection point with 95% confidence intervals
- Validate using multiple statistical approaches (e.g., maximum likelihood estimation)
Application Note: A 2025 study utilizing this method in infants identified an inflection point at 6.83 ng/mL (95% CI: 5.02-8.64), challenging conventional thresholds [15]. This protocol is particularly relevant for establishing bone-health specific thresholds.
Protocol 2: Dose-Response Meta-Analysis for Disease-Specific Targets
Background: This methodology identifies optimal 25(OH)D concentrations for specific health outcomes by synthesizing data from multiple observational studies [9].
Materials and Tools:
- PubMed/MEDLINE database access
- Statistical software with meta-analysis capabilities (e.g., R with metafor package)
- Data extraction forms
- Quality assessment tools (e.g., Newcastle-Ottawa Scale)
Procedure:
- Conduct systematic literature search using predefined terms
- Apply inclusion/exclusion criteria to select studies
- Extract quantitative data on 25(OH)D levels and health outcomes
- Convert all 25(OH)D values to common units (nmol/L or ng/mL)
- Model dose-response relationships using restricted cubic splines or similar methods
- Determine 25(OH)D level at which lowest risk occurs for specific outcome
- Assess heterogeneity and potential sources of bias
- Perform sensitivity analyses to test robustness of findings
Application Note: This approach revealed outcome-specific variations, with optimal levels ranging from approximately 60 nmol/L for cardiovascular disease to 85-100 nmol/L for cancer prevention [9].
Research Reagent Solutions
Table 3: Essential Research Reagents for Vitamin D Status Studies
| Reagent/Kit | Function | Application Notes |
|---|---|---|
| ELISA-based 25(OH)D Detection Kit | Quantifies total 25(OH)D in serum/plasma | Suitable for high-throughput studies; good precision [16] |
| LC-MS/MS Assay for Vitamin D Metabolites | Gold standard for 25(OH)D quantification | Provides highest accuracy; can distinguish D2 and D3 forms |
| Chemiluminescent Immunoassay (CLIA) | Automated 25(OH)D measurement | Used in clinical laboratories; high reproducibility [17] |
| PTH Immunoassay Kit | Measures intact parathyroid hormone | Essential for establishing bone-related thresholds [15] |
| Vitamin D Receptor Antibodies | Detects VDR expression in tissues | Useful for mechanistic studies on tissue-specific responses |
| Custom Software for Dose-Response Modeling | Statistical analysis of threshold effects | Enables inflection point calculation and curve fitting [9] |
Methodological Workflow and Conceptual Framework
Research Workflow for Establishing 25(OH)D Thresholds illustrates the comprehensive approach required to define evidence-based vitamin D thresholds. The process begins with careful population selection, recognizing that factors such as age, ethnicity, health status, and geographic location significantly influence vitamin D metabolism and requirements [16] [15]. The measurement phase requires special attention to assay standardization, as different methodologies can yield variations in 25(OH)D quantification [16] [17]. Contemporary research emphasizes outcome-specific threshold development, moving beyond a one-size-fits-all approach to recognize that optimal levels may differ for fracture prevention versus cancer risk reduction [9]. The critical validation phase requires randomized controlled trials in truly deficient populations, addressing a key limitation of previous studies that often included vitamin D-replete participants [11].
Vitamin D Status Assessment Methodology delineates the laboratory workflow for determining vitamin D status, highlighting critical decision points that impact result interpretation. The choice of assay methodology significantly influences measured 25(OH)D values, with LC-MS/MS generally regarded as the gold standard due to its ability to distinguish between vitamin D2 and D3 metabolites [16] [17]. ELISA methods offer practical advantages for high-throughput studies, while automated CLIA platforms are widely used in clinical laboratories [16] [17]. The interpretation phase requires careful consideration of the appropriate reference standard, recognizing that different thresholds may be applicable based on the clinical or research context [10] [9] [18]. For older adults, this decision is particularly important as musculoskeletal outcomes may require different thresholds than metabolic or immune outcomes [9] [14].
The definition of 25(OH)D sufficiency and deficiency continues to evolve as research reveals the complexity of vitamin D physiology across different populations and health outcomes. The field is moving away from one-size-fits-all thresholds toward a more nuanced understanding that incorporates age-specific, outcome-specific, and population-specific considerations. For older adults, current evidence supports targeting 25(OH)D levels of at least 20-30 ng/mL (50-75 nmol/L) for musculoskeletal benefits, while recognizing that higher levels may be needed for optimal non-skeletal outcomes [9] [12]. Future research should prioritize randomized controlled trials in truly deficient populations, standardized assay methodologies, and exploration of individual factors that influence vitamin D requirements, including genetic polymorphisms in vitamin D metabolism pathways [13] [11]. These advances will enable more personalized approaches to vitamin D supplementation and more effective public health strategies for addressing vitamin D deficiency in vulnerable populations like older adults.
Vitamin D plays a dual role in musculoskeletal health, impacting both bone integrity and muscle function in older adults. Emerging evidence suggests that vitamin D's protective effects against fractures may be mediated more significantly through the prevention of sarcopenia than through direct effects on bone mineral density (BMD) [19]. This paradigm shift has important implications for developing targeted interventions for aging populations. These application notes synthesize current evidence and provide detailed protocols for investigating vitamin D's mechanisms and therapeutic applications in bone health and sarcopenia prevention.
Quantitative Evidence Synthesis
Table 1: Vitamin D Status and Musculoskeletal Health Outcomes
| Health Outcome | Measure | Association Strength | Key Findings |
|---|---|---|---|
| Hip Fracture Risk | Mediation Analysis | 50% mediation | PMI (muscle function) mediated 50% of vitamin D's protective effect vs. 33.3% for FN aBMD [19] |
| Sarcopenia Prevalence | Odds Ratio (OR) | OR = 7.75 | Vitamin D deficiency (<20 ng/mL) associated with 7.75x higher sarcopenia risk (95% CI: 1.96-30.71) [20] |
| Serum 25(OH)D Levels | Inverse Relationship | OR = 0.61 | 25(OH)D >75 nmol/L associated with 39% lower sarcopenia odds vs. <50 nmol/L [21] |
| Muscle Mass & Strength | Percentage Change | +1.9% vs. -3.4% | Active vitamin D (eldecalcitol) increased muscle mass vs. placebo decrease over 1 year [22] |
| Fracture Prevention | Combined Supplementation | 30% risk reduction | Calcium with vitamin D reduced hip fracture risk by 30% (SRRE: 0.70; 95% CI: 0.56-0.87) [23] |
| Study Design | Population | Key Parameters | Outcomes |
|---|---|---|---|
| Retrospective Cohort [19] | 138 hip fracture vs. 182 control patients (age ≥50) | Vitamin D, PMI, FN aBMD | Vitamin D and PMI were independent protective factors against hip fracture |
| NHANES Analysis [21] | 9,489 U.S. adults (2011-2018) | Serum 25(OH)D, folate, sarcopenia (ASM/BMI) | Synergistic interaction between 25(OH)D and folate on sarcopenia risk (RERI significant) |
| RCT - Active Vitamin D [22] | 32 participants, 1-year eldecalcitol vs. placebo | mTOR/FOXO1 pathways, muscle mass/strength | Significant increases in phosphorylation of mTOR and FOXO1 signaling pathways |
| Meta-Analysis [23] | Multiple RCTs of community-dwelling older adults | Vitamin D + calcium supplementation vs. fracture risk | Reduced total fractures by 15% (SRRE: 0.85; 95% CI: 0.73-0.98) |
Experimental Protocols
Protocol: Comprehensive Musculoskeletal Assessment in Aging Populations
Application: Baseline characterization for vitamin D intervention studies
Methodology:
- Participant Recruitment:
Vitamin D Status Assessment:
- Sample: Fasting blood collection at 8:00 AM
- Analysis: Serum 25(OH)D via automated electrochemiluminescence system (Roche)
- Deficiency Definition: <20 ng/mL (50 nmol/L); Insufficiency: 20-30 ng/mL [19]
Muscle Function Evaluation:
- Pectoralis Muscle Index (PMI): Chest CT at T4 level, manual segmentation of pectoralis muscles, PMA calculation (-29 to +150 HU threshold), PMI = PMA/height² (cm²/m²) [19]
- Sarcopenia Diagnosis: EWGSOP2 criteria: low muscle strength (handgrip <27 kg men, <16 kg women), confirmed with low muscle mass (DXA), plus poor physical performance for severe sarcopenia [25]
Bone Health Assessment:
Statistical Analysis:
- Multivariable regression adjusting for age, sex, BMI
- Mediation analysis using "RMediation" package in R [19]
Protocol: Molecular Mechanisms of Active Vitamin D on Muscle Pathways
Application: Preclinical investigation of vitamin D's effects on muscle synthesis/degradation pathways
Methodology:
- Study Design:
- Randomized controlled trial: Active vitamin D (eldecalcitol) vs. placebo for 1 year
- Muscle biopsies pre- and post-intervention [22]
Western Blot Analysis:
- Protein Extraction: Muscle tissue homogenization in RIPA buffer with protease/phosphatase inhibitors
- Antibody Panel:
- Muscle synthesis: p-mTOR, p-p70S6K1, p-rpS6, p-4E-BP1
- Muscle degradation: p-FOXO1, MuRF1
- Normalization: Housekeeping proteins (GAPDH, β-actin) [22]
Body Composition Analysis:
- Method: Bioelectrical impedance analysis (BIA) with body composition analyzer
- Parameters: Skeletal muscle mass, fat mass, muscle strength [22]
Data Analysis:
- Phosphorylation levels compared via ANOVA
- Correlation between pathway activation and muscle mass/strength changes [22]
Signaling Pathways Visualization
Diagram Title: Vitamin D Muscle Synthesis and Degradation Pathways
Diagram Title: Vitamin D Musculoskeletal Research Workflow
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Materials and Analytical Tools
| Item | Function/Application | Example Specifications |
|---|---|---|
| 25(OH)D Assay | Gold standard vitamin D status assessment | Automated electrochemiluminescence system (Roche); LC-MS/MS for reference method [19] [21] |
| DEXA Scanner | Bone mineral density and body composition | Lunar Prodigy dual-energy X-ray bone densitometer (GE Healthcare); precision <1.0% CV [19] [23] |
| CT Imaging | Muscle mass and quality assessment | Six-row spiral CT scanner (Siemens); tube voltage 120 kV, layer thickness 0.625-2 mm [19] |
| 3D Slicer Software | Muscle segmentation and analysis | Open-source platform for PMA calculation; threshold range -29 to +150 HU for skeletal muscle [19] |
| Vitamin D Receptor Antibodies | Muscle VDR expression and localization | Validated for Western blot, IHC; specific to VDR isoforms in muscle tissue [25] [22] |
| Pathway Antibody Panel | Muscle synthesis/degradation signaling | Phospho-specific antibodies: p-mTOR, p-p70S6K1, p-FOXO1; total protein antibodies [22] |
| Active Vitamin D Analogs | Intervention studies | Eldecalcitol; 1α,25-dihydroxyvitamin D3 (calcitriol); dose-range finding required [22] |
Research Gaps and Future Directions
Current evidence supports vitamin D's role in musculoskeletal health, particularly through muscle function preservation, yet several research gaps remain. The optimal serum 25(OH)D concentration for sarcopenia prevention requires clarification, with current evidence suggesting 20-40 ng/mL minimizes fall and fracture risk [26]. The synergistic relationship between vitamin D and other nutrients, particularly folate, presents a promising research avenue [21]. Future studies should establish standardized sarcopenia diagnostic criteria across populations and investigate individual genetic factors affecting vitamin D response and musculoskeletal outcomes.
Molecular mechanisms of active vitamin D analogs represent a critical research frontier, particularly their effects on mTOR and FOXO1 signaling pathways in human muscle [22]. Large-scale clinical trials targeting older adults with vitamin D deficiency and employing multimodal interventions (combining vitamin D with exercise and nutritional optimization) show particular promise for advancing musculoskeletal health in aging populations [19] [25] [20].
The traditional understanding of vitamin D has been fundamentally rooted in its classical role in calcium and phosphate homeostasis. However, emerging research over recent decades has revealed profound extraskeletal effects, particularly in immunomodulation and cellular aging processes. This paradigm shift recognizes vitamin D as a potent immunomodulator with significant implications for autoimmune disease pathogenesis and management, as well as potential influences on biological aging through telomere dynamics. The expression of vitamin D receptors (VDR) on various immune cells—including lymphocytes, macrophages, and dendritic cells—provides the fundamental mechanistic basis for these non-skeletal functions [27]. This application note synthesizes current evidence and methodologies for investigating vitamin D's immunomodulatory properties and its relationship to telomere biology, providing researchers with practical frameworks for advancing this promising field of study.
Molecular Mechanisms of Vitamin D in Immune Regulation
Vitamin D Metabolism and Signaling in Immune Cells
The immunomodulatory effects of vitamin D are primarily mediated through the active metabolite 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) and its nuclear receptor (VDR). Following classical hepatic 25-hydroxylation and renal 1α-hydroxylation, extrarenal production of active vitamin D occurs in various immune cells, including macrophages and dendritic cells, allowing for localized immunoregulation [27] [28]. The VDR functions as a transcription factor that heterodimerizes with the retinoid X receptor (RXR), binding to vitamin D response elements (VDREs) in target gene regulatory regions [27]. This VDR-mediated transcriptional regulation underpins vitamin D's diverse effects on both innate and adaptive immunity.
Mechanisms of Immune Modulation
Vitamin D exerts comprehensive effects across multiple immune cell populations through both genomic and non-genomic pathways:
Innate Immune System Modulation:
- Macrophages/Monocytes: Enhances antimicrobial peptide production (cathelicidin, β-defensin 2), suppresses Toll-like receptor (TLR) 2/4 expression, and reduces pro-inflammatory cytokine production (IL-1, IL-6, TNF-α) [28] [29]
- Dendritic Cells: Inhibits differentiation and maturation, reduces expression of MHC-II, CD40, CD80, and CD86 costimulatory molecules, and promotes tolerogenic phenotype with increased IL-10 and decreased IL-12 production [28]
Adaptive Immune System Modulation:
- T Lymphocytes: Suppresses Th1 and Th17 cell differentiation and cytokine production (IFN-γ, IL-17), while promoting Th2 and regulatory T cell (Treg) responses and enhancing IL-4 and IL-10 production [28] [29]
- B Lymphocytes: Inhibits plasma cell differentiation, class-switched memory B cell formation, and autoantibody production [28]
The following diagram illustrates the key molecular pathways through which vitamin D modulates immune function:
Vitamin D in Autoimmune Diseases: Clinical Evidence and Applications
Epidemiological and Clinical Evidence
Substantial evidence links vitamin D status with autoimmune disease incidence and activity. Epidemiological studies demonstrate notable latitudinal gradients in autoimmune disease prevalence, with higher rates in regions with reduced sunlight exposure [27]. Multiple observational studies consistently report lower serum 25(OH)D levels in patients with various autoimmune conditions compared to healthy controls, with inverse correlations between vitamin D status and disease activity [27].
Key Clinical Associations:
- Multiple Sclerosis: Inverse correlation between vitamin D status and relapse rates, with supplementation (4,000 IU/day) demonstrating reduced MRI lesion burden and clinical activity in relapsing-remitting MS [29]
- Rheumatoid Arthritis: Inverse correlation between vitamin D intake and disease incidence, with noted improvements in disease activity markers with supplementation [27] [30]
- Systemic Lupus Erythematosus: Mendelian randomization studies suggest potential causal protective effects [31]
- Type 1 Diabetes: Vitamin D supplementation in early childhood associated with 30% reduction in disease risk [27]
- Psoriasis: Genetic evidence supports causal protective relationship with vitamin D status [31]
Table 1: Vitamin D in Autoimmune Diseases - Clinical Evidence Summary
| Autoimmune Disease | Evidence Type | Key Findings | Effective Doses in Studies |
|---|---|---|---|
| Multiple Sclerosis | RCT, Observational | Reduced relapse rates, decreased MRI lesions | 4,000 IU/day [29], 100,000 IU biweekly [29] |
| Rheumatoid Arthritis | Observational, Meta-analysis | Inverse correlation with incidence, reduced disease activity | 2,000 IU/day in VITAL [7] |
| Systemic Lupus Erythematosus | Mendelian Randomization | Suggested causal protective effects | N/A [31] |
| Type 1 Diabetes | Observational | 30% risk reduction with childhood supplementation | Variable [27] |
| Psoriasis | Mendelian Randomization | Causal protective relationship | N/A [31] |
| Crohn's Disease | Clinical Trial | 25% decrease in need for infliximab escalation | 20,000 IU/day [29] |
Sex-Specific Considerations in Autoimmunity
An emerging body of evidence highlights significant sex-specific effects in vitamin D immunomodulation, potentially contributing to the female predominance in many autoimmune conditions. Notably, estrogen has been demonstrated to enhance vitamin D function by promoting accumulation and increasing VDR expression, potentially resulting in more potent anti-inflammatory responses in females compared to males [28]. This interaction may be particularly relevant for understanding the sexual dimorphism observed in conditions like multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus.
Vitamin D and Telomere Dynamics: Implications for Cellular Aging
Telomere Biology and Aging
Telomeres are repetitive nucleotide sequences at chromosome ends that protect against degradation and fusion. Telomere shortening occurs naturally with each cell division and is accelerated by oxidative stress and inflammation, serving as a marker of cellular aging [30]. Shorter leukocyte telomere length (LTL) predicts age-related disease incidence and all-cause mortality, making telomere maintenance a key target for healthy aging interventions.
Vitamin D Effects on Telomere Dynamics
Recent large-scale randomized trials provide compelling evidence for vitamin D's role in telomere maintenance:
VITAL Trial Telomere Substudy Findings:
- Design: 1,054 participants aged 50+ (females) and 55+ (males) randomized to vitamin D3 (2,000 IU/day) or placebo for 4 years [7] [32]
- Results: Significant reduction in telomere shortening in vitamin D group compared to placebo, equivalent to approximately 3 years of slowed cellular aging [33] [7] [32]
- Mechanisms: Proposed pathways include reduction of inflammatory burden and oxidative stress on leukocyte precursors [30]
The relationship between vitamin D supplementation and telomere dynamics can be visualized through the following experimental workflow:
Table 2: Vitamin D and Telomere Dynamics - Research Evidence
| Study Design | Population | Intervention | Duration | Key Findings |
|---|---|---|---|---|
| VITAL RCT Substudy [7] [32] | n=1,054, ages 50+ | 2,000 IU/day vitamin D3 vs placebo | 4 years | Significant reduction in telomere shortening, equivalent to ~3 years of slowed aging |
| TwinsUK Cohort [30] | n=2,160 women, ages 18-79 | Observational | Cross-sectional | Positive association between serum vitamin D and LTL after adjustment for age and other covariates |
| TwinsUK Analysis [30] | Tertile comparison | Observational | Cross-sectional | 107 base pair difference in LTL between highest and lowest vitamin D tertiles, equivalent to 5 years of telomeric aging |
Experimental Protocols and Methodologies
Protocol: Assessing Vitamin D Effects on Immune Cell Populations
Objective: To evaluate vitamin D-mediated immunomodulation in human peripheral blood mononuclear cells (PBMCs).
Materials:
- Freshly isolated or cryopreserved human PBMCs
- RPMI-1640 complete medium with 10% FBS
- 1,25(OH)2D3 (calcitriol) stock solution (10⁻⁵ M in ethanol)
- Anti-CD3/CD28 activation beads
- Flow cytometry antibodies: CD4, CD25, CD127, IFN-γ, IL-17, IL-10
- ELISA kits for cytokine quantification (IFN-γ, IL-17, IL-10, IL-4)
- RNA extraction kit and qPCR reagents
Methodology:
- PBMC Isolation and Culture: Isolate PBMCs from whole blood using density gradient centrifugation. Culture at 1×10⁶ cells/mL in complete medium.
- Vitamin D Treatment: Add 1,25(OH)2D3 at physiological (10 nM) and pharmacological (100 nM) concentrations. Include vehicle control (ethanol).
- T Cell Polarization:
- Th1: IL-12 (10 ng/mL) + anti-IL-4 (1 μg/mL)
- Th17: TGF-β (5 ng/mL) + IL-6 (50 ng/mL) + anti-IFN-γ/IL-4
- Treg: TGF-β (10 ng/mL) + IL-2 (100 IU/mL)
- Flow Cytometric Analysis: After 5-7 days, stimulate cells with PMA/ionomycin in presence of brefeldin A for 4-6 hours. Stain for surface markers, then intracellular cytokines.
- Gene Expression Analysis: Extract RNA after 24h stimulation. Analyze VDR target genes (CYP24A1) and cytokine genes by qPCR.
- Statistical Analysis: Compare treated vs. control groups using appropriate statistical tests (paired t-test, ANOVA with post-hoc).
Protocol: Leukocyte Telomere Length Measurement
Objective: To quantify telomere length in peripheral blood leukocytes as a biomarker of cellular aging.
Materials:
- Genomic DNA isolated from whole blood or PBMCs
- Restriction enzymes (HinfI, RsaI)
- TeloTAGGG Telomere Length Assay Kit (Roche) or qPCR reagents
- Southern blot apparatus or real-time PCR system
- Chemiluminescence detection system or SYBR Green master mix
Methodology (Southern Blot):
- DNA Digestion: Digest 2-4 μg genomic DNA with HinfI and RsaI restriction enzymes (10 U/μg each) for 16h at 37°C.
- Gel Electrophoresis: Separate digested DNA on 0.8% agarose gel (25-30V for 16h) alongside molecular weight marker.
- DNA Transfer and Hybridization: Transfer to nylon membrane, hybridize with digoxigenin-labeled telomere-specific probe.
- Detection and Analysis: Detect with anti-digoxigenin-AP and chemiluminescent substrate. Calculate mean terminal restriction fragment (TRF) length using formula: TRF = Σ(ODi)/Σ(ODi/Li) where ODi is signal intensity and L_i is fragment length at position i.
Methodology (qPCR):
- Reaction Setup: Prepare separate reactions for telomere (T) and single-copy gene (S) amplification using validated primers.
- Amplification: Run in triplicate on real-time PCR system with appropriate standards.
- Calculation: Determine T/S ratio using comparative Ct method, convert to kilobase pairs using standard curve.
Quality Control: Include reference DNA samples in each run, maintain inter-assay CV <5%, perform duplicate measurements with <5% difference.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for Vitamin D Immunomodulation Studies
| Reagent/Category | Specific Examples | Research Application | Key Considerations |
|---|---|---|---|
| Vitamin D Metabolites | 1,25(OH)₂D₃ (calcitriol), 25(OH)D₃, Vitamin D₃ | In vitro treatment studies | Solubility (ethanol/DMSO), concentration range (physiological 10⁻⁹-10⁻⁸ M to pharmacological 10⁻⁷ M) |
| VDR Modulators | VDR agonists (calcipotriol), VDR antagonists (TEI-9647) | Mechanistic studies of VDR-specific effects | Selectivity, potency (EC₅₀/IC₅₀), off-target effects |
| Immune Cell Isolation | PBMC isolation kits, CD4⁺ T cell isolation kits, magnetic bead separation | Obtain specific immune cell populations | Purity (>95% for T cell subsets), viability (>90%), activation status |
| Cell Culture Media | RPMI-1640, DMEM with vitamin D-deficient FBS | Controlled vitamin D exposure | Charcoal-stripped FBS to remove hormones, defined media formulations |
| Flow Cytometry Antibodies | CD3, CD4, CD8, CD25, CD127, IFN-γ, IL-17, IL-10, FOXP3 | Immune phenotyping and intracellular cytokine detection | Fluorochrome compatibility, titration for optimal signal-to-noise, intracellular staining protocols |
| Cytokine Detection | ELISA kits, Luminex multiplex arrays, ELISpot kits | Quantify cytokine production | Sensitivity (pg/mL range), dynamic range, cross-reactivity assessment |
| Molecular Biology Tools | VDR siRNA/shRNA, VDR overexpression vectors, VDRE reporter constructs | Manipulate VDR expression and signaling | Transfection efficiency (primary immune cells challenging), specificity of genetic manipulation |
| Telomere Length Assay | TeloTAGGG Kit (Southern blot), qPCR Telomere Length Assay | Cellular aging biomarker | DNA quality/quantity, inter-assay standardization, reference samples |
The accumulating evidence for vitamin D's immunomodulatory properties and potential effects on cellular aging represents a significant expansion of its physiological relevance beyond skeletal health. The mechanistic insights into VDR-mediated immune regulation, combined with clinical evidence from autoimmune diseases and telomere biology, support targeted vitamin D intervention as a promising strategy for immune recalibration and healthy aging.
Future research priorities should include:
- Precision Nutrition Approaches: Stratification by VDR polymorphisms, baseline vitamin D status, and autoimmune disease endotypes
- Optimal Dosing Regimens: Determination of disease-specific and individual-specific dosing for maximal immunomodulation with minimal risk
- Combination Therapies: Investigation of vitamin D as an adjunct to biological therapies in autoimmune conditions
- Long-term Outcomes: Extended follow-up of vitamin D interventions on both autoimmune disease progression and age-related morbidity
- Mechanistic Depth: Further elucidation of the intersection between vitamin D signaling, sex hormones, and immune function
The integration of validated biomarkers—including immune cell phenotypes, cytokine profiles, and telomere length—will be essential for advancing personalized applications of vitamin D in clinical practice and establishing evidence-based supplementation guidelines tailored to older adult populations.
Vitamin D deficiency represents a significant global public health issue, particularly for older adults. This condition impairs calcium and phosphorus homeostasis, leading to the bone hypomineralization disorders osteomalacia and rickets, and has been associated with numerous non-skeletal disorders including infectious diseases, metabolic syndrome, and cognitive impairment [34] [35]. The aging process itself is a recognized risk factor for vitamin D deficiency due to a diminished capacity for dermal synthesis following UVB exposure, reduced kidney activation of vitamin D, and age-related behavioral changes such as decreased outdoor activity [36]. This application note provides a comprehensive analysis of the global prevalence of vitamin D deficiency in older adult cohorts and detailed protocols for assessing vitamin D status in research and clinical settings, framed within the context of developing evidence-based vitamin D supplementation guidelines for older adults.
Global Prevalence and Epidemiological Patterns
Recent meta-analyses of population-based studies from 2000 to 2022, encompassing 7.9 million participants across 81 countries, reveal a substantial global burden of vitamin D deficiency [34] [37]. The prevalence varies significantly based on the threshold used to define deficiency, as detailed in Table 1.
Table 1: Global Prevalence of Vitamin D Deficiency by Diagnostic Threshold
| 25(OH)D Threshold | Classification | Global Prevalence (%) | 95% Credibility Interval |
|---|---|---|---|
| < 30 nmol/L (<12 ng/mL) | Deficiency | 15.7% | 13.7–17.8 |
| < 50 nmol/L (<20 ng/mL) | Deficiency/Insufficiency* | 47.9% | 44.9–50.9 |
| < 75 nmol/L (<30 ng/mL) | Insufficiency/Sufficient | 76.6% | 74.0–79.1 |
Note: Threshold definitions vary by organization. The Institute of Medicine defines <30 nmol/L as deficiency and 30-50 nmol/L as insufficiency, while the Endocrine Society defines ≤50 nmol/L as deficiency [34] [38].
Regional and Demographic Variations
The distribution of vitamin D deficiency demonstrates significant geographical and demographic patterning. A study of older adults in Birjand, Iran, found that 8.42% had vitamin D deficiency (25(OH)D < 12 ng/mL or <30 nmol/L) and 17.06% had insufficient levels (12-20 ng/mL or 30-50 nmol/L), indicating that approximately 25% of this older cohort had suboptimal vitamin D status [36]. Notably, this high prevalence was observed in a sunny region, highlighting that adequate sunlight exposure alone does not guarantee sufficient vitamin D status in older populations.
Table 2: Regional and Demographic Variations in Vitamin D Deficiency Prevalence
| Factor | Subgroup | Prevalence/Association |
|---|---|---|
| Geographical Region | Eastern Mediterranean | Higher prevalence |
| Lower-middle-income countries | Higher prevalence | |
| Seasonality | Winter-Spring | 1.7 times higher than Summer-Autumn |
| Gender | Female | Increased vulnerability |
| Age in Older Adults | 70-79 years vs. 60-69 years | 43% lower chance of deficiency |
| Body Weight | Overweight/Obese | Protective (OR = 0.36, 0.35 respectively) |
| Supplement Use | Vitamin D supplementation | Protective (OR = 0.31) |
Research from South India revealed an even more alarming prevalence, with 58% of adults aged ≥45 years exhibiting vitamin D deficiency (<20 ng/mL), 23% insufficient (20-29 ng/mL), and only 19% with normal levels (≥30 ng/mL) [39]. This study also identified a significant association between vitamin D deficiency and dyslipidemia, with 91.2% of vitamin D deficient individuals also having dyslipidemia. After adjusting for covariates, individuals with deficient vitamin D levels demonstrated lower scores in ACE-III verbal fluency compared to those with normal levels (p = 0.038), suggesting a potential link between vitamin D status and cognitive performance [39].
Experimental Protocols for Vitamin D Status Assessment
Protocol 1: Serum 25-Hydroxyvitamin D Measurement
Principle: Serum 25-hydroxyvitamin D [25(OH)D] is the most reliable clinical indicator of vitamin D status, representing the major circulating form of vitamin D and integrating both cutaneous synthesis and dietary intake [39].
Specimen Collection and Handling:
- Collect venous blood samples after at least 12-hour overnight fasting
- Process samples within 2 hours of collection
- Centrifuge at 6000 rpm for serum separation
- Store serum at ≤ -70°C until analysis
- Avoid repeated freeze-thaw cycles
Analysis Methods:
- Primary Method: Chemiluminescence immunoassay on systems such as VITROS ECiQ Immunodiagnostic System using Intellicheck Technology [39]
- Alternative Methods:
- Enzyme-Linked Immunosorbent Assay (ELISA) using commercial kits
- Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) for reference methodology
- Colorimetry methods using autoanalyzers (e.g., Prestige 24i, Rome, Italy)
Quality Assurance:
- Implement internal quality controls with each batch
- Participate in external proficiency testing programs
- Validate methods against reference standards
- Document intra-assay and inter-assay coefficients of variation
Diagram 1: Vitamin D Status Assessment Workflow
Protocol 2: Population-Based Survey Methodology
Study Design:
- Cross-sectional or longitudinal designs with population-based sampling
- Multistage stratified cluster random sampling to ensure representativeness
- Sample size calculation to achieve sufficient statistical power
- Ethical approval and informed consent acquisition
Data Collection:
- Demographic Data: Age, gender, education, employment, smoking status
- Medical History: Chronic diseases (diabetes, hypertension, CKD, COPD), medication history
- Lifestyle Factors: Physical activity assessment using standardized questionnaires (e.g., LASA Physical Activity Questionnaire)
- Nutritional Status: Mini Nutritional Assessment (MNA) questionnaire
- Anthropometric Measurements: Height, weight, BMI according to NHANES protocol
- Seasonal Variation: Document season of blood collection (critical for interpretation)
Statistical Analysis:
- Standardize prevalence estimates according to reference populations (e.g., WHO 2000-2025)
- Employ appropriate statistical tests (Kruskal-Wallis H test for non-normally distributed variables)
- Use chi-square tests for categorical variables
- Perform multivariate logistic regression to identify independent determinants
- Calculate odds ratios with 95% confidence intervals
- Consider survey analysis techniques to account for complex sampling designs
Vitamin D Status Classification and Clinical Implications
The interpretation of vitamin D status varies slightly among expert organizations, leading to different prevalence estimates based on the classification system employed. Diagram 2 illustrates the decision pathway for classifying vitamin D status according to major guidelines.
Diagram 2: Vitamin D Status Classification Pathways
Health Implications in Older Adults
Vitamin D deficiency in older adults is associated with several significant health outcomes:
- Skeletal Health: Increased risk of osteomalacia, osteoporosis, and fractures [34] [35]
- Muscle Function: Reduced muscle strength, increased fall risk [38]
- Critical Illness Outcomes: In critically ill pediatric patients, vitamin D deficiency was associated with increased mortality (OR 2.05) and greater need for inotropic support (OR 2.02) [40]
- Cognitive Function: Potential associations with cognitive performance, particularly verbal fluency [39]
- Metabolic Health: Strong associations with dyslipidemia and other metabolic disorders [39]
Notably, the U.S. Preventive Services Task Force (USPSTF) recommends against supplementation with vitamin D with or without calcium for the primary prevention of fractures and against supplementation with vitamin D for the prevention of falls in community-dwelling postmenopausal women and men age 60 years or older, concluding with moderate certainty that supplementation has no net benefit for these outcomes [24]. This highlights the complexity of translating vitamin D status into clinical practice and the need for further research.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for Vitamin D Status Assessment
| Reagent/Equipment | Specifications | Application/Function |
|---|---|---|
| 25(OH)D Assay Kits | Chemiluminescence immunoassay (e.g., VITROS ECiQ) | Quantitative measurement of serum 25(OH)D levels |
| ELISA Kits | 25-OH vitamin D kits (e.g., Padtan Gostar Isar) | Alternative method for 25(OH)D quantification |
| Blood Collection Tubes | Serum separation tubes | Standardized blood sample acquisition |
| Centrifuge | Capable of 6000 rpm | Serum separation from whole blood |
| Ultra-Low Freezer | ≤ -70°C capacity | Long-term serum sample preservation |
| Autoanalyzer Systems | Prestige 24i, Ap22 Speedy | Automated analysis of multiple samples |
| Calibration Standards | Manufacturer-provided | Assay calibration and quality control |
| Quality Control Materials | Low, medium, high 25(OH)D concentrations | Intra- and inter-assay precision monitoring |
Vitamin D deficiency remains highly prevalent in older adult populations globally, with approximately 47.9% of the general population having 25(OH)D levels below 50 nmol/L, the threshold many experts consider deficient [34]. This high prevalence persists despite variations by geography, season, gender, and age. The substantial burden of vitamin D deficiency warrants public health attention, particularly given the aging global population and the potential impact on bone health, metabolic function, and overall quality of life in older adults.
Standardized assessment protocols using reliable methods for 25(OH)D measurement are essential for accurately determining prevalence and evaluating interventions. The mixed evidence regarding supplementation benefits highlights the need for further research to clarify optimal vitamin D status for different older adult subpopulations and to develop targeted, effective public health strategies to reduce the burden of vitamin D deficiency in this vulnerable demographic.
Translating Evidence into Practice: Dosage, Formulations, and Monitoring Protocols
Analysis of International Guideline-Recommended Daily Intakes (RDI) for Adults >70 Years (400-2000 IU/day)
Vitamin D sufficiency is critical for maintaining musculoskeletal health in older adults, a population particularly vulnerable to deficiency due to reduced cutaneous synthesis and dietary intake. The role of vitamin D extends beyond calcium homeostasis to potential effects on fall prevention, cognitive function, and overall mortality. This application note analyzes international guideline-recommended daily intakes (RDI) for adults over 70 years, which range from 400 to 2000 IU/day, and provides experimental protocols for investigating vitamin D efficacy in this demographic. The variability in recommendations reflects ongoing scientific debate regarding optimal serum 25-hydroxyvitamin D (25(OH)D) levels and supplementation strategies for achieving maximal health benefits in the geriatric population. Understanding the evidence base supporting these guidelines is essential for researchers developing clinical trials and therapeutic interventions targeting age-related vitamin D insufficiency.
Comprehensive Analysis of International Guidelines
Clinical practice guidelines for vitamin D supplementation in older adults exhibit significant variation, reflecting differing interpretations of available evidence and regional practices. A systematic review of evidence-based clinical guidelines published from 2013 to 2024 identified 31 guidelines for analysis [41]. The review found that while no guideline recommended screening or supplementation for the general adult population, specific recommendations existed for older adults and other at-risk groups.
Table 1: International Guideline Recommendations for Adults >70 Years
| Organization/Entity | Region | Recommended Daily Intake for Adults >70 | Key Population Notes | Serum 25(OH)D Target |
|---|---|---|---|---|
| Institutes of Medicine (IOM) | North America | 800 IU (20 mcg) [42] [43] | General healthy population | 50 nmol/L (20 ng/mL) or above [42] |
| Bone Health and Osteoporosis Foundation (BHOF) | North America | 800-1,000 IU daily [44] | Adults age 50 and older | Not specified |
| Endocrine Society (2024) | International | Higher than IOM RDA (specific dose not defined) [12] | Adults over 75 for potential mortality reduction | Not specified; against routine testing [12] |
| American Geriatrics Society | USA | 1000 IU daily [41] | All adults ≥ 65 years for fall prevention | 75 nmol/L [41] |
| Nordic Nutrition Recommendations | Nordic Countries | 800 IU daily [41] | General population | 50 nmol/L [41] |
| European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) | Europe | 800-1000 IU/d [41] | People at risk, including elderly | 50-75 nmol/L [41] |
The 2024 Endocrine Society guideline represents a significant evolution in recommendations, suggesting that adults over 75 may benefit from supplementation above the IOM recommended daily allowance for potential mortality reduction, but notably recommends against routine testing for vitamin D levels in healthy populations [12]. This reflects an ongoing paradigm shift toward targeted rather than universal supplementation approaches.
Critical Appraisal of Evidence Base
Fall Prevention Efficacy
The most compelling evidence for vitamin D supplementation in older adults exists for fall prevention, with dosage and administration frequency identified as critical factors. A 2024 network meta-analysis of 35 randomized controlled trials (RCTs) involving 58,937 participants provided nuanced insights [45]:
Table 2: Vitamin D Dosage and Fall Risk Based on Meta-Analysis Evidence
| Daily Vitamin D Dose | Fall Risk Compared to Placebo | Key Contextual Factors |
|---|---|---|
| 400 IU or less | No fracture prevention benefit [43] | Inadequate for therapeutic effect |
| 800-1000 IU | 15% lower risk (RR = 0.85, 95%CI: 0.74-0.95) [45] | Optimal range for fall prevention |
| >1000 IU | Increased risk compared to 800-1000 IU/d [45] | Potential for harm at very high doses |
| 500-800 IU | Reduced hip and non-spine fractures by ~20% [43] | Benefit shown in meta-analysis |
| 2000 IU | No reduction in fractures or falls [43] | VITAL trial findings in generally healthy adults |
This analysis revealed that daily administration of 800-1000 IU vitamin D was associated with a 22% reduction in fall risk (RR = 0.78, 95%CI:0.64-0.92), whereas intermittent administration showed no preventive effect [45]. Furthermore, the benefit was significant only in individuals with baseline 25(OH)D levels ≤ 50 nmol/L, highlighting the importance of targeting deficient populations.
A feasibility study specifically investigating older adults ≥70 years with low vitamin D levels and recent fall history found that 800 IU/day supplementation for 6 months significantly increased blood vitamin D levels (from 23.25±4.8 ng/ml to 29.13±6.9 ng/ml; p<0.001) and reduced self-reported falls (from 3.76±2.2 to 0.76±1.4 falls; p<0.0001) [46].
Non-Musculoskeletal Outcomes
Evidence for benefits beyond musculoskeletal health remains emerging. A retrospective study of older adults with hypertension and mild cognitive impairment suggested that high-dose supplementation (5000 IU/day) was associated with improvements in recognition memory and systolic blood pressure [47]. However, the Endocrine Society's 2024 guideline found insufficient evidence to support vitamin D supplementation for non-musculoskeletal conditions in generally healthy populations [12] [10].
Experimental Protocols for Vitamin D Research
Protocol: Fall Reduction Efficacy Study
Objective: To evaluate the effect of 800-1000 IU/day vitamin D3 supplementation on fall incidence in adults >70 years with baseline vitamin D insufficiency (25(OH)D <30 ng/mL).
Population:
- Inclusion: Adults ≥70 years; 25(OH)D <30 ng/mL; slow gait speed (<1.2 m/s); ≥2 falls in previous year
- Exclusion: Disorders affecting vitamin D metabolism (CKD, hyperparathyroidism); medications interfering with vitamin D; malabsorption syndromes [46]
Intervention:
- Experimental: 800-1000 IU vitamin D3 (cholecalciferol) once daily for 6 months
- Control: Matching placebo
- Co-interventions: All participants receive calcium (500-600 mg/day) if dietary intake insufficient [45]
Outcome Assessment:
- Primary: Change in fall rate (prospective daily fall diaries)
- Secondary: Short Physical Performance Battery (SPPB), handgrip strength, Timed Up and Go (TUG), 6-minute walk test [46]
- Biochemical: Serum 25(OH)D, calcium, PTH at baseline and 6 months
Monitoring: Pill counts and adherence diaries (target: ≥80% adherence); safety monitoring for hypercalcemia [46] [47]
Protocol: Cognitive Function Substudy
Objective: To assess whether vitamin D supplementation improves cognitive function in older adults with hypertension and mild cognitive impairment.
Population:
- Inclusion: Age ≥65 years; hypertension; 25(OH)D <30 ng/mL; MoCA <26 or subjective cognitive complaints
- Exclusion: Neurological disorders; severe renal/hepatic impairment; baseline calcium >10.5 mg/dL [47]
Intervention:
- Experimental: 5000 IU/day vitamin D2 (ergocalciferol) for ≥6 months
- Control: No supplementation or <800 IU/day
- Note: This high-dose protocol applies only to deficient populations in research settings [47]
Outcome Assessment:
- Primary: Recognition memory (Hopkins Verbal Learning Test-Revised)
- Secondary: Global cognition (MoCA), systolic and diastolic BP [47]
- Biochemical: 25(OH)D, calcium, creatinine at baseline and 6 months
Analysis: Multiple regression adjusting for age, baseline MoCA, antihypertensive medications [47]
Molecular Signaling Pathways
Research Reagent Solutions
Table 3: Essential Research Reagents for Vitamin D Clinical Studies
| Reagent/Category | Specific Examples | Research Application | Technical Notes |
|---|---|---|---|
| Vitamin D Formulations | Cholecalciferol (D3), Ergocalciferol (D2) | Intervention studies | D3 preferred for supplementation studies; pharmacy-grade for trials [46] [47] |
| 25(OH)D Immunoassays | ELISA, CLIA, RIA | Status assessment | Prefer LC-MS/MS for reference method; consistent assay across study [46] [47] |
| Functional Assessment Tools | Short Physical Performance Battery (SPPB), Handgrip Dynamometer | Muscle function outcomes | Standardized administration critical; assess fall risk [46] |
| Cognitive Assessment | Montreal Cognitive Assessment (MoCA), Hopkins Verbal Learning Test-Revised | Cognitive outcomes | MoCA <26 indicates mild impairment [47] |
| Fall Monitoring | Prospective fall diaries, Morse Fall Scale | Primary outcome measurement | Daily recording reduces recall bias [46] |
| Calcium Homeostasis Markers | Serum calcium, PTH, creatinine | Safety and mechanism | Monitor for hypercalcemia, especially with high-dose therapy [47] |
Discussion and Research Gaps
Despite comprehensive guideline development, significant knowledge gaps persist regarding optimal vitamin D supplementation for older adults. The 2024 systematic review by Bischoff-Ferrari et al. noted that "further research is needed to get more conclusive data to get a better understanding of the effects of vitamin D deficiency and the benefit of a sufficient vitamin D level to generate standardized evidence-based recommendations" [41].
Critical research priorities include:
- Dose-response optimization: Determining whether >1000 IU/day provides additional benefits for specific subpopulations without increasing fall risk [45]
- Baseline status influence: Confirming whether effects are limited to deficient individuals (25(OH)D <50 nmol/L) as suggested by meta-analyses [45]
- Formulation differences: Comparative effectiveness of vitamin D2 versus D3 in older adults, particularly at higher doses [47]
- Non-skeletal outcomes: Rigorous trials investigating cognitive, cardiovascular, and immunomodulatory effects in appropriately powered studies [47]
- Administration frequency: Systematic evaluation of daily versus intermittent dosing regimens across different clinical outcomes [45]
The move toward personalized supplementation strategies based on baseline status, genetic factors, and specific health conditions represents the next frontier in vitamin D research for geriatric populations.
International guidelines recommend vitamin D supplementation ranging from 400-2000 IU/day for adults over 70 years, with 800-1000 IU/day emerging as the optimal range for fall prevention in deficient populations. The evidence strongly supports daily—not intermittent—administration targeted at older adults with documented vitamin D insufficiency (25(OH)D <50 nmol/L). Higher doses (>1000 IU/day) require careful consideration as they may increase fall risk in some populations. Future research should focus on precision medicine approaches to identify individuals most likely to benefit from supplementation beyond current recommended daily intakes, particularly for non-skeletal outcomes. Standardized experimental protocols and comprehensive molecular pathway analysis will be essential for advancing our understanding of vitamin D's pleiotropic effects in the aging population.
Clinical Evidence for High-Dose Vitamin D in Rheumatoid Arthritis
Recent clinical studies and meta-analyses provide compelling evidence for the use of high-dose vitamin D supplementation (4000 IU/day) in managing Rheumatoid Arthritis (RA). The data demonstrates significant improvements in both clinical disease activity scores and key inflammatory biomarkers.
Table 1: Clinical Outcomes of High-Dose Vitamin D Supplementation in RA
| Outcome Measure | Reported Effect Size | Statistical Significance | Study Design | Citation |
|---|---|---|---|---|
| Disease Activity Score-28 (DAS-28) | WMD: -0.83 (95% CI: -1.38 to -0.28) [48] | p < 0.001 [48] | Systematic Review & Meta-Analysis | [48] |
| Disease Activity Score-28 (DAS-28) | Significant reduction (U'=2285.5) [49] | P < 0.0001 [49] | Prospective RCT (4000 IU/day) | [49] |
| Pain Visual Analog Scale (VAS) | Significant pain reduction (U'=2245.5) [49] | P < 0.0001 [49] | Prospective RCT (4000 IU/day) | [49] |
| Pain Visual Analog Scale (VAS) | SMD = -1.54 (95% CI: -2.53, -0.55) [50] | P = 0.002 [50] | Systematic Review & Meta-Analysis | [50] |
| C-Reactive Protein (CRP) | WMD: -0.24 (95% CI: -0.45 to -0.03) [48] | p = 0.03 [48] | Systematic Review & Meta-Analysis | [48] |
| C-Reactive Protein (CRP) | SMD = -0.88 (95% CI: -1.31, -0.44) [50] | P = 0.001 [50] | Systematic Review & Meta-Analysis | [50] |
| Erythrocyte Sedimentation Rate (ESR) | WMD: -4.08 (95% CI: -4.67 to -3.50) [48] | p < 0.001 [48] | Systematic Review & Meta-Analysis | [48] |
| Serum Vitamin D Level | WMD: +12.69 ng/mL (95% CI: 1.80 to 23.59) [48] | p = 0.02 [48] | Systematic Review & Meta-Analysis | [48] |
A 2025 prospective, randomized, double-blind study specifically investigated a 4000 IU/day dosage over six months. This trial found significant reductions in both pain (VAS score) and disease activity (DAS-28 score) compared to a control group, confirming the therapeutic potential of this specific high-dose regimen [49]. The certainty of evidence for these outcomes, particularly for improved serum vitamin D levels, is graded as moderate to high [48].
Detailed Experimental Protocol for 4000 IU/day Supplementation
The following protocol is synthesized from a 2025 clinical trial investigating high-dose vitamin D in RA patients [49].
Study Design and Participant Selection
- Trial Design: Prospective, randomized, parallel-group, double-blind, placebo-controlled trial with a 6-month follow-up period.
- Participants:
- Cohort: 100 RA patients (82 women, 18 men).
- Diagnostic Criteria: Must fulfill the 2010 ACR/EULAR classification criteria for RA [49].
- Disease Duration: 1 to 14 years.
- Age Range: 30-65 years.
- Key Exclusion Criteria:
- Presence of other inflammatory diseases (e.g., Crohn's disease, ulcerative colitis).
- Thyroid or parathyroid diseases.
- Liver or kidney diseases.
- Treatment in the past 3 months with calcium >1 g/day or vitamin D supplements.
Intervention and Blinding
- Intervention Group: Oral administration of 4000 IU vitamin D3 (cholecalciferol) once daily after breakfast. The study used capsules manufactured by Erbozeta S.r.l., San Marino [49].
- Control Group: Placebo or standard care without vitamin D supplementation.
- Concomitant Medication: No changes to existing RA medications (e.g., DMARDs) were permitted during the follow-up period to ensure stability.
Data Collection and Outcome Measures
Primary Endpoints (Assessed at baseline and 6 months):
- Disease Activity: Disease Activity Score-28 (DAS-28), using CRP as the inflammatory marker.
- Pain Intensity: Visual Analogue Scale (VAS) for overall pain.
Secondary Endpoints (Assessed at baseline and 6 months):
- Inflammatory Biomarkers:
- Serum C-Reactive Protein (CRP)
- Erythrocyte Sedimentation Rate (ESR)
- Interleukin-6 (IL-6)
- Interleukin-17 (IL-17)
- Tumour Necrosis Factor-alpha (TNF-α)
- Vitamin D Status: Serum 25-hydroxyvitamin D (25(OH)D). Baseline levels were confirmed to be <20 ng/mL to ensure deficiency/hyposufficiency [49].
- Immunological Markers: Anti-citrullinated peptide (ACPA) antibodies.
Statistical Analysis
- Non-parametric tests (Mann-Whitney U test) were used for comparing VAS and DAS-28 scores due to the non-normal distribution of data.
- A p-value of <0.05 was considered statistically significant.
Mechanistic Pathways of High-Dose Vitamin D in RA
Vitamin D supplementation modulates RA pathophysiology through several key immunologic pathways, with high doses (4000 IU/day) potentially exerting more potent effects.
The primary mechanisms involve:
- Immunomodulation: Active vitamin D (1,25(OH)₂D) suppresses the differentiation and proliferation of pro-inflammatory T-helper 17 (Th17) cells, which play a central role in RA pathogenesis by producing IL-17 [50]. Concurrently, it promotes the development of regulatory T-cells (Tregs) that help maintain immune tolerance [51].
- Cytokine Inhibition: By modulating these immune cells, high-dose vitamin D significantly reduces the production of key inflammatory cytokines, including IL-17, TNF-α, and IL-6 [49] [50]. This leads to downstream reductions in acute phase reactants like CRP and ESR.
- Bone Health Preservation: RA-associated inflammation accelerates bone loss. By promoting calcium absorption and bone metabolism, vitamin D helps mitigate the osteoporotic risk common in RA patients [49] [52].
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents and Materials for Clinical Research
| Item/Category | Specific Example & Details | Research Function |
|---|---|---|
| Vitamin D Supplement | Vitamin D3 (Cholecalciferol), 4000 IU capsules. Erbozeta S.r.l. [49] | The active pharmaceutical ingredient (API) for the intervention arm. |
| Placebo | Matched capsule identical in appearance to the vitamin D capsule. | Control intervention to maintain blinding and assess the true effect of vitamin D. |
| Clinical Assessment Kits | Disease Activity Score-28 (DAS-28) proforma. Visual Analog Scale (VAS) for pain (0-100 mm). | Quantification of primary clinical endpoints for disease activity and pain. |
| Immunoassay Kits | ELISA kits for: IL-6, IL-17, TNF-α, ACPA. | Measurement of specific inflammatory mediators and autoantibodies to elucidate mechanism of action. |
| Clinical Chemistry Analyzers | Automated platforms for CRP, ESR, and 25-hydroxyvitamin D [25(OH)D] analysis. | Measurement of standardized inflammatory biomarkers and confirmation of vitamin D status. |
| Data Management Software | Statistical software packages (e.g., Stata, R). | For meta-analysis and data analysis, including handling of heterogeneity (I² statistics) [48] [50]. |
Application Notes for Protocol Implementation
- Dosage Safety: The selected 4000 IU/day dosage is considered safe and effective for adult RA populations, as established in prior dose-response studies [49]. The upper tolerable limit is typically set at 4000 IU/day [53].
- Heterogeneity Considerations: Researchers should note significant heterogeneity (I² > 80%) often observed in meta-analyses for outcomes like VAS and DAS-28 [50]. Stratify recruitment or plan subgroup analyses based on ethnicity, baseline vitamin D status, and supplementation duration to account for this.
- Context in Older Adult Research: These findings align with the broader thesis that vitamin D supplementation requires a targeted, condition-specific approach. While general population studies like those from the USPSTF find no net benefit for fracture prevention in healthy community-dwelling older adults [24], this protocol demonstrates a clear therapeutic role in a specific inflammatory disease context. This underscores that vitamin D is not a universal supplement but a targeted therapeutic agent for specific pathological states like RA.
Vitamin D deficiency remains a significant public health concern, particularly in older adult populations, due to age-related reductions in cutaneous synthesis, decreased sun exposure, and nutritional challenges [54]. This document provides a structured framework for evaluating two primary supplementation strategies: daily low-dose administration and intermittent high-dose (depot) regimens. Evidence from randomized controlled trials (RCTs) and meta-analyses indicates that while daily dosing often produces a marginally superior pharmacokinetic profile for raising serum 25-hydroxyvitamin D (25(OH)D) concentrations [55] [56], intermittent administration serves as an effective alternative, particularly in populations where adherence to daily therapy is problematic [55] [57] [58]. The selection of a regimen must therefore balance efficacy with practical considerations of adherence and feasibility, especially in older adults and institutionalized settings. These application notes consolidate current evidence and provide detailed experimental protocols to guide clinical research and drug development in geriatric nutrition.
Table 1: Summary of Key RCT Findings Comparing Vitamin D Supplementation Regimens
| Study Population | Intervention & Dose | Duration | Change in Serum 25(OH)D | Key Comparative Findings | Citation |
|---|---|---|---|---|---|
| Nursing Home Residents (Mean age: 84 yrs) | 600 IU/day vs. 4200 IU/week vs. 18,000 IU/month | 4 months | Daily: 69.9 nmol/LWeekly: 67.2 nmol/LMonthly: 53.1 nmol/L | Daily dose was more effective than weekly, and monthly was the least effective. All raised 25(OH)D levels. | [56] |
| Newly Settled Refugee Children (Aged 0-16 yrs) | Daily vs. Depot therapy (doses based on 25(OH)D levels) | 40 weeks | Daily group had significantly higher 25(OH)D at each post-baseline visit. | Both groups achieved sufficiency. Daily group had a higher proportion >50 nmol/L at all time points. | [55] [59] |
| Network Meta-Analysis (116 RCTs) | Various daily vs. intermittent frequencies with equivalent total doses | Variable | No statistically significant pooled mean differences between daily and intermittent with similar dosage. | Efficacy of intermittent supplementation was similar to daily. Weekly 600,000 IU/3 months had highest efficacy. | [57] |
| Psychogeriatric Nursing Home Residents | 5,600 IU/week (capsule) vs. 7,500 IU/week (drops) | >3 months | Capsules: 90 nmol/LDrops: 41 nmol/L | Formulation significantly impacted efficacy, despite equivalent nominal dosing. | [60] |
Table 2: Impact of Regimen on Non-Skeletal Outcomes in Older Adults
| Outcome | Impact of Daily Low-Dose Regimen | Impact of Intermittent High-Dose (Boluses) Regimen | Consensus/Evidence Level |
|---|---|---|---|
| Falls Risk | Associated with a reduction in fall risk in the elderly. | Infrequent, large bolus doses may increase the risk of falls. | Expert Consensus [54] |
| Fracture Risk | Reduced fracture risk when combined with calcium, as demonstrated in frail older females. | Evidence for fracture reduction with intermittent dosing alone is less established. | Clinical Trial & Consensus [54] |
| Progression to T2DM | Supplementation may reduce the risk of progression from prediabetes to type 2 diabetes. | Specific effect of intermittent dosing on diabetes progression is less defined. | Expert Consensus [54] |
Detailed Experimental Protocols
Protocol for a Randomized Controlled Trial Comparing Regimens
Objective: To compare the efficacy of daily low-dose versus monthly high-dose oral vitamin D3 supplementation in raising and maintaining serum 25(OH)D concentrations >50 nmol/L in older adults (>65 years) residing in long-term care facilities.
1. Study Design:
- Type: Prospective, parallel-group, randomized controlled trial.
- Blinding: Double-blind. The monthly group receives an active monthly dose + daily placebo, while the daily group receives active daily doses + monthly placebo.
- Duration: 6 months, with a possible extension to 12 months to assess long-term maintenance.
2. Participant Selection:
- Inclusion Criteria: Age >65 years, residency in a participating long-term care facility, life expectancy >12 months, and serum 25(OH)D level <50 nmol/L at screening.
- Exclusion Criteria: Hypercalcemia; severe renal or hepatic impairment; current use of vitamin D supplements (>400 IU/day) or drugs interfering with vitamin D metabolism (e.g., anticonvulsants, systemic corticosteroids); history of kidney stones; and terminal illness.
3. Randomization and Intervention:
- Randomization: Stratified by baseline 25(OH)D level (<30 nmol/L vs. 30-50 nmol/L) and gender. A computer-generated sequence will allocate participants in a 1:1 ratio.
- Intervention Groups:
- Daily Group (n=100): Oral vitamin D3 (cholecalciferol) 1,600 IU per day. This dose is chosen to account for the higher need in this deficient, frail population and to align with recommendations suggesting 2000 IU/day for sufficiency [61].
- Monthly Group (n=100): Oral vitamin D3 48,000 IU once per month (equivalent to ~1,600 IU/day).
4. Outcome Measures and Assessment Schedule: Table 3: Assessment Schedule and Key Metrics
| Assessment | Baseline | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|
| Serum 25(OH)D | X | X | X | X |
| Serum PTH | X | X | X | X |
| Serum Calcium, Albumin, Phosphate | X | X | X | X |
| Physical Performance (e.g., SPPB) | X | - | X | X |
| Falls & Fracture Incidents | - | Monthly | Monthly | Monthly |
| Adherence (Pill Count) | - | X | X | X |
5. Statistical Analysis:
- Primary Outcome: Mean change in serum 25(OH)D concentration from baseline to 6 months.
- Analysis: Intention-to-treat analysis using linear mixed models to examine longitudinal changes over time, controlling for confounders such as age, gender, BMI, baseline 25(OH)D, and sun exposure score [55]. A per-protocol analysis will also be conducted.
Protocol for Serum 25(OH)D and Biomarker Analysis
1. Sample Collection:
- Collect non-fasting venous blood samples in serum separator tubes.
- Process samples within 2 hours of collection by centrifugation at 2000-3000 RCF for 15 minutes.
- Aliquot serum into cryovials and store at -80°C until analysis to prevent degradation.
2. Biochemical Assays:
- Serum 25-Hydroxyvitamin D [25(OH)D]:
- Recommended Method: Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS). This is considered the gold standard for specificity and accuracy, capable of distinguishing between 25(OH)D2 and 25(OH)D3.
- Alternative Methods: Commercially available immunoassays (e.g., DiaSorin Liaison) can be used, but laboratories should establish method-specific reference ranges and be aware of potential cross-reactivity issues [55] [56].
- Secondary Biomarkers:
- Parathyroid Hormone (PTH): Measured by electrochemiluminescence immunoassay (ECLIA) to assess physiological response to vitamin D supplementation.
- Albumin-Corrected Serum Calcium: Measured by colorimetric assay.
- Additional Markers: Alkaline Phosphatase (ALP) and Phosphate.
3. Data Quality Control:
- All samples from a single participant should be analyzed in the same batch to minimize inter-assay variation [56].
- Use internal quality controls and participate in external quality assurance/proficiency testing (e.g., DEQAS).
Signaling Pathways and Metabolic Workflow
Diagram 1: Vitamin D Metabolism and Activity Pathway. This pathway illustrates the metabolic activation of vitamin D from sources like sunlight, diet, and supplements, culminating in its biological effects on calcium homeostasis, bone health, and other physiological functions.
Diagram 2: Clinical Trial Workflow for Regimen Comparison. This workflow outlines the key stages of a clinical trial designed to compare daily versus intermittent vitamin D supplementation regimens, from initial setup to final analysis.
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Research Materials and Reagents
| Item/Category | Specification & Function | Example Application/Note |
|---|---|---|
| Vitamin D3 (Cholecalciferol) | Form: Pharmaceutical-grade oral solutions (oily or aqueous) or capsules. Function: The active supplement compound. | Ensure stability and bioavailability. Bio-Logical Vitamin D3 solution (5000 IU/mL) used in RCTs [55]. Formulation (capsule vs. drop) can critically impact efficacy [60]. |
| Placebo | Form: Matched in appearance, taste, and packaging to the active supplement. Function: Serves as a control in blinded trials. | Typically composed of the carrier (e.g., olive oil, medium-chain triglyceride oil) without active vitamin D. |
| LC-MS/MS System | Equipment: Liquid Chromatography with Tandem Mass Spectrometry. Function: Gold-standard method for quantifying serum 25(OH)D and 25(OH)D2/D3 separately. | Provides high specificity and accuracy compared to immunoassays. |
| Automated Immunoassay System | Equipment: e.g., DiaSorin Liaison, Abbott Architect. Function: High-throughput measurement of serum 25(OH)D, PTH, and other biomarkers. | More accessible but requires cross-validation with LC-MS/MS. Used in multiple major RCTs [55] [56]. |
| Serum Biobank | Materials: Cryogenic vials, -80°C Freezers. Function: Long-term storage of patient serum samples for batch analysis and future studies. | Maintains sample integrity for longitudinal and secondary analysis. |
| Validated Questionnaires | Tools: Sun Exposure Score (SES) [55], Dietary Calcium/Vitamin D Intake (3-day food diary), Falls Calendar. Function: Quantifies non-supplement sources of vitamin D and records clinical outcome events. | Critical for controlling confounders like sun exposure and diet in statistical models. |
The Role of Supervised Loading Doses and Adherence Strategies in Clinical Practice
Vitamin D deficiency remains a significant public health concern, particularly among older adults, with profound implications for musculoskeletal health and fall prevention. Current recommended dietary allowances (RDAs) of 600-800 IU per day are often insufficient to correct deficiency in a timely manner, leaving an unmet medical need for effective repletion strategies. This application note evaluates the critical role of supervised loading-dose protocols as a safe and effective method to rapidly normalize serum 25-hydroxyvitamin D (25(OH)D) levels in deficient older adults. Emerging evidence indicates that conventional supplementation approaches frequently fail to achieve target concentrations (>30 ng/mL) associated with optimal skeletal and muscle function outcomes. Furthermore, recent meta-analyses have demonstrated that untargeted vitamin D supplementation provides no fall risk reduction in community-dwelling older adults, highlighting the necessity for precision medicine approaches based on individual deficiency status and tailored dosing regimens.
The protocols outlined herein provide clinical researchers and drug development professionals with evidence-based methodologies for implementing effective loading-dose strategies while mitigating potential risks associated with high-dose supplementation. By integrating pharmacokinetic principles, safety monitoring parameters, and adherence-enhancing protocols, these approaches address fundamental challenges in vitamin D repletion therapy for geriatric populations. The strategic implementation of supervised loading regimens represents a paradigm shift from population-wide supplementation to targeted deficiency correction, potentially optimizing clinical outcomes while minimizing resource utilization in an era of increasingly restrictive testing guidelines.
Current Evidence and Quantitative Analysis
Efficacy of Vitamin D Supplementation in Older Adults
Recent high-quality evidence has fundamentally reshaped our understanding of vitamin D supplementation in older adult populations. A comprehensive 2025 meta-analysis of 10 randomized controlled trials (RCTs) involving 23,211 community-dwelling adults aged ≥65 years found no significant association between vitamin D supplementation and reduced fall risk (OR = 0.99; 95%CI: 0.95–1.03) [62] [63]. This null effect was consistent across multiple subgroups and dosing regimens:
Table 1: Vitamin D Supplementation and Fall Risk in Older Adults [62] [63]
| Subgroup | Number of Studies | Odds Ratio (95% CI) | I² Heterogeneity |
|---|---|---|---|
| Overall | 10 | 0.99 (0.95-1.03) | 31% |
| Women only | 6 | 0.97 (0.92-1.02) | 31.2% |
| Men only | 4 | 1.08 (0.98-1.20) | 0% |
| Dose ≤1000 IU/day | 5 | 0.96 (0.90-1.02) | 39.5% |
| Dose >1000 IU/day | 5 | 1.02 (0.96-1.09) | 0% |
| Duration ≤12 months | 6 | 0.96 (0.90-1.02) | 56.2% |
| Duration >12 months | 4 | 1.01 (0.96-1.07) | 0% |
These findings have prompted major guideline organizations to recommend against routine vitamin D testing and supplementation in the general population, instead advocating for targeted supplementation in specific high-risk groups, including adults ≥75 years, where decreased skin synthesis and intestinal absorption create heightened vulnerability to deficiency [41] [13].
Loading Dose Efficacy and Safety Profile
In contrast to maintenance dosing, loading-dose regimens demonstrate significantly enhanced efficacy in rapidly correcting vitamin D deficiency. A 2024 post-hoc evaluation compared three supplementation protocols in deficient patients, revealing substantial differences in treatment effectiveness [64]:
Table 2: Comparative Efficacy of Vitamin D Loading Dose Protocols [64]
| Protocol | Total Dose | Duration | Daily Equivalent | Mean Δ 25(OH)D (ng/mL) | Dose-Response Ratio (ng/mL/100 IU) |
|---|---|---|---|---|---|
| Moderate-Faster Loading | 300,000 IU | 5 weeks | 8,571 IU | +31.04 ± 12.95 | 0.36 |
| Slower Loading | 300,000 IU | 10 weeks | 4,286 IU | +25.08 ± 10.16 | 0.59 |
| Low-Dose Maintenance | 84,000 IU | 12 weeks | 1,000 IU | +11.44 ± 6.15 | 1.15 |
The moderate-faster loading protocol (60,000 IU weekly for 5 weeks) achieved target 25(OH)D levels (>30 ng/mL) in all deficient subjects regardless of body mass index (BMI), while the slower loading protocol showed attenuated efficacy in overweight and obese subgroups (p < 0.0014) [64]. Safety analysis demonstrated no significant differences in hypercalcemia or hypercalciuria incidence between loading and low-dose protocols, supporting the favorable risk-benefit profile of supervised loading regimens.
Experimental Protocols
Vitamin D Loading Dose Protocol for Deficient Older Adults
Patient Stratification and Inclusion Criteria
This protocol employs a double-blind, randomized, controlled design to evaluate the efficacy and safety of loading doses in vitamin D-deficient adults ≥65 years. Participant selection follows strict criteria:
- Inclusion: Serum 25(OH)D <20 ng/mL (moderate deficiency) or <12 ng/mL (severe deficiency); community-dwelling adults ≥65 years; capacity to provide informed consent.
- Exclusion: Baseline hypercalcemia (serum calcium >10.5 mg/dL); estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m²; history of nephrolithiasis; malabsorption syndromes; current use of thiazide diuretics or antiepileptic medications; contraindications to vitamin D supplementation.
Intervention Arms and Dosing Regimens
Eligible participants are stratified by BMI category (<25 kg/m², 25-30 kg/m², >30 kg/m²) and randomized to one of three intervention arms:
- Arm A (Moderate-Faster Loading): 60,000 IU cholecalciferol weekly for 5 weeks (total 300,000 IU), followed by maintenance dosing of 2,000 IU daily.
- Arm B (Slower Loading): 30,000 IU cholecalciferol weekly for 10 weeks (total 300,000 IU), followed by maintenance dosing of 2,000 IU daily.
- Arm C (Standard Maintenance): 1,000 IU cholecalciferol daily for 12 weeks (total 84,000 IU), followed by maintenance dosing of 1,000 IU daily.
All supplements are administered as oral cholecalciferol (D3) with the largest meal of the day to enhance absorption, as co-ingestion with dietary fat increases serum 25(OH)D concentrations by approximately 50% compared to fasting administration [65].
Safety Monitoring and Dose Adjustment
Rigorous safety monitoring is implemented throughout the intervention period:
- Baseline assessment: Comprehensive metabolic panel (including serum calcium, albumin, creatinine), 25(OH)D, parathyroid hormone (PTH), urinary calcium-to-creatinine ratio.
- Week 5-10 monitoring: Serum calcium and 25(OH)D levels measured at mid-intervention point for loading dose arms.
- Week 12 endpoint assessment: Repeat of all baseline measurements plus adverse event documentation.
- Stopping rules: Protocol suspension criteria include serum calcium >11.0 mg/dL, 25(OH)D >100 ng/mL, or urinary calcium-to-creatinine ratio >0.37.
The following diagram illustrates the complete experimental workflow:
Adherence Enhancement Strategies
Structured Supervision Protocol
Optimizing adherence to vitamin D regimens requires multidimensional strategies:
- Directly observed therapy (DOT): Weekly administration supervised by healthcare personnel or designated caregivers during the loading phase, transitioning to electronic medication adherence monitoring (e.g., smart blister packs, connected pill bottles) during maintenance.
- Educational reinforcement: Structured counseling on administration timing (with largest meal) and the rationale for loading dose intensity, emphasizing the non-linear dose-response relationship where higher doses produce disproportionately greater increases in 25(OH)D levels.
- Reminder systems: Automated text messaging or mobile application notifications for self-administered doses, with escalation protocols for missed doses (>24 hours late).
Pharmaceutical Formulation Considerations
Selection of appropriate vitamin D formulations significantly impacts treatment success:
- Cholecalciferol (D3) preference: Superior efficacy compared to ergocalciferol (D2), with studies demonstrating D3 produces 5-9 times greater potency in raising and sustaining 25(OH)D levels due to higher affinity for vitamin D binding protein and more efficient 25-hydroxylation [65].
- Oil-based preparations: Enhanced bioavailability compared to powder-filled capsules, particularly when administered with dietary fat.
- Fixed-dose combinations: With elemental calcium (500-1200 mg/day) for patients with osteoporosis, as recommended by clinical guidelines for fracture prevention [41].
Mechanistic Pathways and Physiological Response
Vitamin D Metabolism and Calcium Homeostasis
Understanding the pharmacokinetic and pharmacodynamic principles underlying loading dose regimens is essential for protocol optimization. The following diagram illustrates key metabolic pathways and their relationship to dosing strategies:
Safety Considerations for High-Dose Protocols
While loading doses demonstrate favorable safety profiles, specific populations warrant heightened vigilance:
- Geriatric vulnerability: Age-related reductions in renal function and potential alterations in vitamin D metabolism necessitate conservative dosing in frail elderly ≥75 years.
- BMI-dependent dosing: Obese individuals (BMI >30 kg/m²) require 2-3 times higher doses to achieve equivalent 25(OH)D responses due to sequestration in adipose tissue [64].
- Bolus dose precautions: The German Federal Institute for Risk Assessment (BfR) cautions against consumer self-administration of high bolus doses (>20,000 IU) due to risk of misuse and potential association with increased fall risk, recommending such regimens be restricted to medically supervised contexts [66].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Materials for Vitamin D Loading Dose Studies
| Reagent/Equipment | Specification | Research Application |
|---|---|---|
| Cholecalciferol Standards | USP-grade cholecalciferol (D3) in oil-based capsules (1,000 IU, 2,000 IU, 50,000 IU) | Gold standard intervention; ensures formulation consistency and bioavailability |
| LC-MS/MS System | Liquid chromatography-tandem mass spectrometry with deuterated internal standards (e.g., d6-25(OH)D3) | Reference method for precise 25(OH)D quantification; essential for pharmacokinetic analysis |
| Automated Immunoassay | Chemiluminescence platforms (Diasorin Liaison, Roche Elecsys, Siemens Centaur) | High-throughput clinical measurement of 25(OH)D; suitable for large-scale trials |
| Vitamin D Binding Protein | Human VDBP ELISA kits | Investigation of free vs. bound vitamin D fractions; particularly relevant in obesity research |
| CYP2R1 and CYP27B1 Assays | Recombinant enzyme activity kits | Mechanistic studies of vitamin D metabolism; identification of genetic polymorphisms affecting response |
| Calcium Homeostasis Panel | Colorimetric assays for serum/urinary calcium, PTH, FGF-23, bone turnover markers (CTX, P1NP) | Comprehensive safety and efficacy monitoring; detection of potential adverse effects |
The strategic implementation of supervised loading doses represents a sophisticated approach to vitamin D repletion that addresses the limitations of conventional supplementation strategies. The documented failure of untargeted vitamin D supplementation to reduce fall risk in community-dwelling older adults underscores the necessity for precision nutrition approaches that prioritize deficient individuals and employ optimized dosing regimens. The protocols outlined herein provide a robust methodology for rapidly correcting deficiency while maintaining vigilant safety monitoring.
Successful translation of these research protocols into clinical practice requires careful consideration of several implementation factors. First, patient stratification based on baseline 25(OH)D status, BMI, and renal function enables personalized dosing optimization. Second, adherence support systems must be integrated throughout the treatment course, particularly during the transition from supervised loading to self-administered maintenance therapy. Finally, appropriate monitoring protocols should be established to detect rare adverse events while avoiding unnecessary testing that contributes to healthcare costs without clinical benefit.
As guideline organizations increasingly restrict routine vitamin D testing, the development of effective, efficient repletion strategies becomes paramount. Supervised loading doses offer a promising solution that balances rapid efficacy with conscientious safety monitoring, potentially optimizing outcomes for vitamin D-deficient older adults while promoting responsible resource utilization. Future research should focus on validating these protocols in diverse clinical settings and examining their impact on hard endpoints beyond fall risk, including fracture incidence, physical function, and quality of life measures.
For researchers and clinicians investigating vitamin D supplementation, particularly in older adult populations, the accurate measurement and interpretation of serum 25-hydroxyvitamin D (25(OH)D) is a critical methodological cornerstone. As the major circulating form of vitamin D, 25(OH)D reflects contributions from both cutaneous synthesis and nutritional intake, serving as the best indicator of overall vitamin D status [67]. This protocol outlines standardized approaches for monitoring this key biomarker, framed within the context of evolving clinical guidelines and evidence from recent large-scale studies.
Current research landscapes must reconcile new evidence with established practice. The 2024 Endocrine Society Clinical Practice Guideline, a key reference for many studies, notably updates its position by focusing on disease prevention in populations without established indications for testing and does not endorse specific serum 25(OH)D targets for healthy adults [10] [68]. This shift underscores the importance of rigorous, context-driven biomarker monitoring in clinical research.
Establishing Vitamin D Status: Reference Intervals and Definitions
Despite its widespread use, the interpretation of serum 25(OH)D levels is challenged by a lack of universal consensus on reference intervals, with different expert bodies proposing varying thresholds.
Table 1: Commonly Referenced 25(OH)D Status Categories
| Status Category | Serum 25(OH)D Level | Notes and Associated Physiological Changes |
|---|---|---|
| Deficiency | < 20 ng/mL (< 50 nmol/L) | Consensus threshold used by WHO, IOM, and EFSA [67] [68] [69]. |
| Insufficiency | 21–29 ng/mL (52–72 nmol/L) | Based on observed changes in calcium absorption and rising PTH levels [67]. |
| Sufficiency | ≥ 30 ng/mL (≥ 75 nmol/L) | Threshold suggested by some societies for optimal bone and muscle function [67]. |
| Toxicity | > 150 ng/mL (> 374 nmol/L) | Level associated with clinical toxicity [67]. |
The Evolving Debate on Optimal Levels
The definition of optimal 25(OH)D status for non-skeletal health outcomes remains an active area of research. A 2025 summary of dose-response meta-analyses suggested that the lowest risk for various diseases, including certain cancers and all-cause mortality, is associated with levels in the range of 75–125 nmol/L (30–50 ng/mL) [9]. This highlights the potential for outcome-specific optimal ranges, which must be considered when designing clinical trials.
Critical Considerations for Assay Methodology
A significant source of variability in 25(OH)D measurement is the laboratory method employed. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) is considered the criterion standard [67]. However, various antibody-based methods (e.g., RIA, ELISA, chemiluminescent assays) are also in widespread use. The Vitamin D External Quality Assurance Scheme (DEQAS) exists to monitor inter-laboratory variability, but researchers must be aware that differences in methodology can impact results and complicate cross-study comparisons [67]. A 2025 study from Barcelona further emphasized that reference intervals can be population- and assay-specific, advocating for the use of big data and indirect methods like the refineR algorithm to establish more accurate, local RIs [68].
Protocol for Monitoring 25(OH)D After Initiation of Supplementation
A standardized protocol for follow-up testing is essential for assessing patient adherence and the biochemical efficacy of the supplementation regimen.
Recommended Timing for Follow-up Testing
The endogenous half-life of 25(OH)D is approximately two weeks in circulation [68]. Therefore, a follow-up measurement is recommended 2 to 3 months after initiating or significantly altering a vitamin D supplementation dose. This interval allows sufficient time for serum levels to stabilize at a new steady state, providing a reliable assessment of the intervention's effect [69]. Real-world analyses confirm that this timeframe is used in clinical practice to evaluate the response to supplementation and adjust doses accordingly [69].
Monitoring Workflow and Clinical Decision-Making
The following diagram illustrates the logical workflow for initiating supplementation and conducting follow-up monitoring.
Interpreting the Response to Supplementation
A real-world study provides a framework for categorizing patient response to maintenance doses of vitamin D, which is crucial for adherence assessment and dose-finding in clinical research [69].
Table 2: Categorizing Response to Vitamin D Supplementation (Maintenance Dose)
| Response Category | Supplementation Dose | Observed Δ in 25(OH)D | Interpretation and Potential Actions |
|---|---|---|---|
| Inadequate/Weak | > 800 IU/day | Increase of ≤ 10 ng/mL | Suggests poor adherence, malabsorption, or other metabolic factors. Consider supervised dosing. |
| Inadequate/Weak | Any dose | Increase within assay precision (≤7%) | Strongly indicates non-adherence or severe malabsorption. |
| Inadequate/Strong | ≤ 800 IU/day | Increase of > 10 ng/mL | Unexpectedly robust response; verify dosing, consider lower maintenance dose. |
| Adequate | Any dose | Increase consistent with dose | Desired response; continue current regimen. |
Factors Influencing Supplementation Efficacy
Research indicates that a significant subset of patients may not achieve target levels with conventional dosing. A 2025 real-world analysis found that 59% of patients on maintenance doses alone had an inadequate response, with 36.3% of cases remaining unexplained even after accounting for known risk factors [69]. This underscores the complexity of vitamin D metabolism and highlights the necessity of monitoring to individualize therapy. Key factors to consider include:
- Adherence: A critical and often variable factor [69].
- Body Fat Percentage: Vitamin D is fat-soluble and can be sequestered in adipose tissue.
- Malabsorption Syndromes: Conditions like celiac disease, Crohn's disease, etc.
- Season: Levels can fluctuate due to variations in sun exposure [70] [68].
The Scientist's Toolkit: Key Research Reagents and Materials
Table 3: Essential Reagents and Materials for 25(OH)D Research
| Item | Function/Application | Technical Notes |
|---|---|---|
| Serum/Plasma Samples | Matrix for 25(OH)D quantification | Gold-standard sample type; stable if handled properly. |
| LC-MS/MS System | Criterion standard method for quantifying 25(OH)D2 and D3. | Provides high specificity and sensitivity; allows for separate metabolite measurement [67]. |
| Immunoassay Kits (e.g., ELISA, CLIA) | High-throughput alternative for 25(OH)D quantification. | Ensure cross-reactivity for both D2 and D3 is understood; participate in DEQAS for standardization [67]. |
| Vitamin D Standard Reference Materials (SRM) | Calibration and method validation. | Sourced from NIST (e.g., SRM 972a) for traceability and standardization across labs. |
| DEQAS Participation | External Quality Assessment (EQA) | Monitors and ensures the reliability and accuracy of 25(OH)D measurements over time [67]. |
Research Context: Key Evidence and Guidelines in Older Adults
When framing research within the context of older adults, several recent guidelines and large-scale studies are essential to reference:
- USPSTF (2024 Draft Recommendation): Recommends against vitamin D supplementation for the primary prevention of fractures and falls in community-dwelling, asymptomatic adults aged 60 years and older, concluding with moderate certainty that there is no net benefit [24]. This highlights a key population and outcome set for researchers to consider.
- Endocrine Society (2024 Guideline): Focuses on the use of vitamin D for disease prevention in individuals without established indications for vitamin D treatment, moving away from specific serum targets for healthy adults [10].
- Systematic Review of Guidelines (2025): Confirms that no major guideline recommends population-wide screening or supplementation for the general adult population. Supplementation is typically reserved for at-risk groups (e.g., osteoporosis, older adults), with common dosages of 400-1000 IU/day and target thresholds of ≥ 50-75 nmol/L [71].
- Regression Dilution in Cohort Studies: A critical methodological consideration for researchers analyzing prospective data. The association between baseline 25(OH)D and health outcomes (e.g., stroke, cognitive decline) weakens with longer follow-up periods due to changes in vitamin D status over time. This can lead to significant underestimation of true associations in long-term studies [72].
Addressing Clinical Challenges and Suboptimal Response in Older Patients
The phenomenon of the "inadequate responder" presents a significant challenge in clinical management of vitamin D status, particularly among older adults. This population demonstrates a high prevalence of vitamin D deficiency, with studies indicating that 24-47% of older adults exhibit serum 25-hydroxyvitamin D (25(OH)D) levels below 50 nmol/L, a threshold considered necessary for bone health [73] [38]. Despite widespread supplementation protocols, a substantial subset of this population fails to achieve therapeutic target levels, representing the inadequate responder profile. Within multimorbid older populations with polypharmacy, research reveals that approximately 33.9% experience potential underuse (lack of supplementation despite clear indications), while 10.2% experience potential overuse (supplementation without clear indications) [73]. This clinical conundrum necessitates systematic investigation into the mechanistic underpinnings of suboptimal vitamin D response and standardized protocols for identification and management.
The inadequate responder is broadly defined as an individual who fails to achieve serum 25(OH)D levels above 50 nmol/L despite daily supplementation with at least 800-1000 IU of vitamin D, the dose recommended by the International Osteoporosis Foundation for older adults [38]. The investigation of these cases requires a multifaceted approach that considers malabsorption syndromes, genetic polymorphisms in metabolic pathways, drug-nutrient interactions, and environmental factors that collectively contribute to treatment resistance. This protocol outlines standardized procedures for identifying, evaluating, and managing inadequate responders within geriatric populations, with particular emphasis on research applications in pharmaceutical development and clinical trial design.
Pathophysiological Mechanisms and Contributing Factors
Key Determinants of Vitamin D Status
Table 1: Major Factors Contributing to Inadequate Vitamin D Response
| Category | Specific Factors | Proposed Mechanism |
|---|---|---|
| Malabsorption | Inflammatory bowel disease, celiac disease, bariatric surgery | Reduced absorption of fat-soluble vitamins |
| Drug Interactions | Anticonvulsants, glucocorticoids, antiretrovirals | Enhanced catabolism via CYP450 induction |
| Genetic Factors | CYP2R1, CYP27B1, GC, VDR polymorphisms | Altered hydroxylation, transport, or receptor binding |
| Environmental | Endocrine-disrupting chemicals (PFAS, PBDEs, phthalates) | Competitive receptor binding and metabolic disruption |
| Age-Related Changes | Reduced skin thickness, decreased sun exposure, renal impairment | Diminished synthesis and activation |
Molecular Interference Pathways
Emerging evidence indicates that endocrine-disrupting chemicals (EDCs) significantly interfere with vitamin D homeostasis through multiple pathways. Recent research demonstrates that mixtures of EDCs, including per- and polyfluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDEs), and organophosphate esters (OPEs), are associated with altered vitamin D biomarker concentrations in children, suggesting potential lifelong disruption patterns [74]. These chemicals may act through competitive receptor binding at the vitamin D receptor (VDR), alteration of vitamin D transport proteins, or modulation of hydroxylation enzymes CYP27B1 and CYP24A1. In adult populations, these disruptions may manifest as apparent treatment resistance, particularly in individuals with higher cumulative EDC exposures.
Diagnostic Protocol for Inadequate Responders
Biochemical Assessment Algorithm
A comprehensive diagnostic evaluation is essential for characterizing inadequate responders. The recommended assessment protocol includes:
Confirmatory Testing: Obtain two separate measurements of serum 25(OH)D after at least 3 months of documented adherence to supplementation (800-1000 IU/day). Use liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodology for highest accuracy [75] [74].
Metabolic Panel: Measure calcium, phosphate, albumin, creatinine with eGFR, and parathyroid hormone (PTH) to assess functional vitamin D status and calcium homeostasis. Elevated PTH despite supplementation suggests functional deficiency.
Malabsorption Screening: Check serum vitamin A simultaneously, as co-existing fat-soluble vitamin deficiencies strengthen the case for malabsorption [75]. Consider celiac serology and fecal elastase in appropriate clinical contexts.
Inflammatory Markers: Assess C-reactive protein (CRP) and interleukin-6 (IL-6) as systemic inflammation upregulates catabolic enzymes. Studies show inverse correlations between IL-6 and 25(OH)D levels in geriatric populations with infections [75].
Clinical Characterization
Document comorbid conditions associated with altered vitamin D metabolism, including:
- Osteoporosis or fragility fractures (present in 33.9% of potentially undertreated older adults) [73]
- Renal impairment (eGFR <60 mL/min/1.73m² reduces 1α-hydroxylation capacity)
- Hepatic dysfunction (impairs 25-hydroxylation)
- Medications known to induce CYP450 isoenzymes (phenytoin, carbamazepine, rifampin)
- Immune conditions (sepsis, autoimmune disorders) associated with consumption
Table 2: Laboratory Assessment Protocol for Inadequate Responders
| Test | Target Population | Interpretation Guidance | Methodology Recommendations |
|---|---|---|---|
| Serum 25(OH)D | All inadequate responders | <50 nmol/L confirms inadequate response | LC-MS/MS preferred for accuracy |
| PTH | All inadequate responders | Elevated level indicates functional deficiency | Two-site immunoassay |
| Calcium Profile | All inadequate responders | Rule out hypercalcemia, assess homeostasis | Spectrophotometry (corrected for albumin) |
| Vitamin A | Suspected malabsorption | Co-deficiency supports malabsorption diagnosis | LC-MS/MS |
| Creatinine/eGFR | All older adults | <60 mL/min suggests impaired activation | Enzymatic method |
| Inflammatory Markers (CRP, IL-6) | Suspected inflammatory consumption | Elevated levels suggest inflammatory sequestration | Immunoassay |
Experimental Design for Mechanistic Investigation
Clinical Protocol for Absorption Studies
To differentiate malabsorption from hypermetabolism, implement a supervised vitamin D challenge test:
Baseline Measurements: After an 8-hour fast, obtain baseline 25(OH)D, 1,25(OH)₂D, and 24,25(OH)₂D₃ levels.
Administration: Administer a single oral dose of 50,000 IU vitamin D₃ with a standardized high-fat meal (30% fat content) to optimize absorption.
Serial Monitoring: Measure 25(OH)D levels at 4, 8, 12, 24, 72, and 168 hours post-administration.
Kinetic Analysis: Calculate area under the curve (AUC), maximum concentration (Cmax), and time to maximum concentration (Tmax).
Interpretation: Normal response shows 25(OH)D increase of ≥25 nmol/L above baseline at 24 hours. Suboptimal increase suggests malabsorption; normal initial rise with rapid decline suggests accelerated catabolism.
Advanced Biomarker Profiling
For research applications, comprehensive metabolic profiling provides mechanistic insights:
Vitamin D Metabolite Ratio (VMR): Calculate 24,25(OH)₂D₃:25(OH)D₃ ratio as an indicator of vitamin D catabolic flux. A ratio >0.05 suggests appropriate metabolic regulation, while lower ratios may indicate saturation of enzymatic capacity or alternative clearance pathways [74].
Free 25(OH)D Assessment: In conditions altering vitamin D binding protein (e.g., liver disease, nephrotic syndrome), measure free 25(OH)D levels via equilibrium dialysis or calculated estimates.
Genetic Profiling: Screen for polymorphisms in CYP2R1, CYP27B1, CYP24A1, GC, and VDR genes associated with altered vitamin D metabolism and signaling.
Research Reagent Solutions
Table 3: Essential Research Reagents for Vitamin D Response Studies
| Reagent/Category | Specific Examples | Research Application |
|---|---|---|
| LC-MS/MS Standards | 25(OH)D₂, 25(OH)D₃, 24,25(OH)₂D₃, 1,25(OH)₂D₃ | Gold-standard quantification of vitamin D metabolites |
| Immunoassays | Intact PTH, IL-6, procalcitonin kits | Assessment of functional status and inflammatory context |
| Cell-Based Reporter Systems | VDR-responsive luciferase constructs | Screening for receptor-binding disruptors and agonists |
| EDC Panels | PFAS, PBDEs, phthalate metabolites | Exposure assessment in epidemiological studies |
| Genotyping Arrays | CYP2R1, CYP27B1, CYP24A1, GC, VDR SNPs | Identification of genetic contributors to poor response |
Therapeutic Approaches for Inadequate Responders
Protocol Optimization Strategies
Based on the characterized phenotype, implement targeted interventions:
For Malabsorption Confirmation:
- Switch to water-miscible formulations or emulsified preparations
- Consider ultraviolet B light therapy (personalized dosing based on Fitzpatrick skin type)
- Administer with the largest meal of the day containing dietary fats
For Enhanced Catabolism:
- Increase supplementation frequency to daily administration (avoiding bolus dosing)
- Consider dose escalation (2,000-4,000 IU daily) with close monitoring for toxicity
- Address modifiable factors such as concomitant medications when possible
For Severe Resistance:
- Consider calcifediol (25(OH)D) supplementation, which bypasses the hepatic hydroxylation step
- In renal impairment, consider active vitamin D analogs (calcitriol, alfacalcidiol) with careful monitoring
Monitoring and Safety Protocol
Implement close safety monitoring with any dose escalation:
Biochemical Monitoring: Check serum calcium, 25(OH)D, and creatinine at 1-month, 3-month, and quarterly intervals during dose adjustment.
Toxicity Thresholds: Maintain 25(OH)D levels <125 nmol/L to avoid potential hypercalcemia and hypercalciuria, with lower thresholds (<100 nmol/L) in patients with renal impairment or calcium-containing kidney stones.
Clinical Assessment: Monitor for symptoms of hypercalcemia (polyuria, polydipsia, nausea, cognitive changes) at each follow-up.
Research Implications and Future Directions
The systematic investigation of inadequate responders to vitamin D supplementation represents a critical frontier in nutritional science and geriatric pharmacology. Current evidence gaps include:
Standardized Definitions: Development of consensus criteria for "inadequate response" across diverse populations.
Biomarker Validation: Establishment of the vitamin D metabolite ratio as a clinical tool for monitoring metabolic flux.
Environmental Impact: Quantification of EDC exposure thresholds that clinically significantly impact vitamin D status.
Personalized Dosing: Development of algorithms incorporating genetic, clinical, and environmental factors to predict individual dosage requirements.
Future clinical trials should prioritize inclusion of inadequate responders as a distinct subgroup, with targeted interventions based on characterized resistance mechanisms. Pharmaceutical development should focus on novel delivery systems that bypass absorption barriers and compounds resistant to accelerated catabolism.
Quantitative Evidence on Risk Factors for Vitamin D Treatment Failure
Epidemiological and clinical trial data reveal distinct patient populations and clinical scenarios that significantly increase the risk of inadequate vitamin D response. The tables below summarize key quantitative findings on prevalence and associated risk factors.
Table 1: Prevalence of Inappropriate Vitamin D Supplementation in Multimorbid Older Adults [73]
| Parameter | Overall Population (n=2008) | Stratified Findings |
|---|---|---|
| Vitamin D Supplementation Rate | 41.1% (825/2008) | - |
| Potential Underuse (High-risk without supplementation) | 33.9% (681/2008) | 69.7% of non-users |
| Potential Overuse (Supplementation without high-risk condition) | 10.2% (204/2008) | 24.7% of users |
| Key Risk Factors for Underuse | - | Male sex, Increasing age, Fewer medications |
| Key Risk Factors for Overuse | - | Higher number of medications |
Table 2: Key Determinants of Profound Vitamin D Deficiency [76]
| Determinant | Impact on Profound Deficiency Risk (25OHD <10 nmol/L) |
|---|---|
| Ethnicity (Asian vs. White in UK Biobank) | 9% prevalence vs. much lower in white population |
| Ambient UVB Radiation (Lowest vs. Highest Quartile) | 17-fold increased risk |
| Vitamin D Supplement Use | 4.4-fold reduced risk |
| Fish Intake | 5-fold reduced risk |
Experimental Protocols for Investigating Vitamin D Treatment Failure
Protocol 1: Assessing Vitamin D Status and Treatment Efficacy in High-Risk Cohorts
Objective: To evaluate the prevalence of vitamin D deficiency and identify factors associated with treatment failure in high-risk populations (e.g., older, multimorbid adults). [73]
Study Population:
- Inclusion Criteria: Multimorbidity (≥3 chronic conditions), polypharmacy (≥5 chronic medications), age ≥70 years. [73]
- High-Risk Conditions: Defined per START criteria (version 2): E2) long-term systemic corticosteroid therapy, known osteoporosis/osteopenia; E3) previous fragility fractures; E5) housebound/nursing home residents or experiencing falls. [73]
Methodology:
- Design: Multicenter, cross-sectional analysis or prospective cohort.
- Data Collection:
- Documentation: Record all diagnoses (ICD-10 codes) and medications (ATC codes), including over-the-counter vitamin D. [73]
- Biochemical Analysis: Measure serum 25-hydroxyvitamin D [25(OH)D] as the primary status biomarker. [77] [76]
- Deficiency Definitions:
- Additional Measures: Parathyroid Hormone (PTH), serum calcium, albumin. [77]
- Outcome Definitions:
- Statistical Analysis: Use multivariable logistic regression (e.g., mixed-effects models) to identify factors (age, sex, medication count, hospitalizations) associated with underuse and overuse. [73]
Protocol 2: Evaluating High-Dose Vitamin D Supplementation in Resistant Populations
Objective: To determine the efficacy and safety of higher-dose vitamin D3 (cholecalciferol) regimens in populations with obesity, malabsorption, or polypharmacy. [78] [79]
Study Population:
- Target Groups: Adults with obesity (BMI ≥30 kg/m²), malabsorption syndromes (e.g., IBD, bariatric surgery), chronic liver/kidney disease, polypharmacy, age >75 years. [78]
Intervention & Dosing Regimens:
- Prevention/Maintenance (without 25(OH)D monitoring):
- Treatment (without initial 25(OH)D monitoring, for 6-8 weeks only):
- Control/Standard Dose: Daily dose of 600-800 IU Vitamin D3. [77]
Methodology:
- Design: Randomized controlled trial.
- Duration: 8-12 weeks for initial repletion; longer (e.g., 1 year) for maintenance studies.
- Primary Endpoint: Proportion of patients achieving serum 25(OH)D level ≥30 ng/mL (75 nmol/L). [78]
- Secondary Endpoints:
- Monitoring: Measure 25(OH)D, calcium, and PTH at baseline, 3 months, and annually if on long-term high-dose therapy. [77]
Pathway to Vitamin D Treatment Failure
The following diagram illustrates the interconnected mechanisms through which key risk factors contribute to vitamin D treatment failure.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents and Assays for Vitamin D Research
| Reagent/Assay | Function/Application |
|---|---|
| LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry) | Gold-standard method for precise quantification of 25-hydroxyvitamin D [25(OH)D] and its metabolites in serum/plasma. Distinguishes between D2 and D3 forms. [76] |
| Immunoassays (ELISA, CLIA) | High-throughput, automated platforms for measuring total 25(OH)D concentration in large-scale epidemiological studies and routine clinical monitoring. [77] |
| Cholecalciferol (Vitamin D3) & Ergocalciferol (Vitamin D2) | Primary supplement forms used in clinical trials. Cholecalciferol is generally preferred for its higher efficacy in raising 25(OH)D levels. [77] [78] |
| Parathyroid Hormone (PTH) Immunoassay | Critical for assessing functional vitamin D status and diagnosing secondary hyperparathyroidism, a key consequence of deficiency. [77] [80] |
| Calcium and Phosphate Colorimetric/Kits | For monitoring calcium homeostasis and detecting potential hypercalcemia, especially in high-dose supplementation studies. [77] |
| Vitamin D Receptor (VDR) Antibodies | Essential for Western Blot, Immunohistochemistry, or flow cytometry to study VDR expression and distribution in target tissues. |
| CYP Enzymes (e.g., CYP2R1, CYP27B1, CYP24A1) | Key enzymes in vitamin D metabolism. Recombinant enzymes or inhibitors are used to study metabolic pathways and drug interactions. [77] [81] |
Patient adherence, defined as the extent to which a person's behavior corresponds with agreed recommendations from a healthcare provider, is a critical determinant of treatment success. Non-adherence to medication generates an estimated €80–125 billion in avoidable costs in the European Union and is associated with nearly 200,000 deaths annually [82]. This challenge is particularly acute among vulnerable elderly populations and for preventive regimens like vitamin D supplementation, where adherence rates remain suboptimal despite clear clinical guidelines [83] [84]. This application note synthesizes current real-world data on supplementation gaps and evaluates the efficacy of educational interventions, providing researchers with structured protocols to address adherence challenges in clinical studies.
Quantitative Data on Supplementation Gaps and Adherence
Global Burden of Non-Adherence
Non-adherence to therapeutic regimens represents a pervasive challenge across healthcare systems worldwide. Approximately 50% of patients do not take their medications as prescribed, with about 30% failing to fill their first prescription [85]. The decline in adherence is progressive; for every 100 prescriptions written, only 50-70% are filled, 48-66% are picked up, 25-30% are taken as prescribed, and merely 15-20% are refilled as directed [85]. In the United States alone, medication non-adherence leads to approximately 125,000 preventable deaths annually [85].
Table 1: Economic and Clinical Impact of Medication Non-Adherence
| Region | Estimated Annual Deaths | Economic Impact | Primary Contributing Factors |
|---|---|---|---|
| European Union | Nearly 200,000 | €80-125 billion in avoidable costs | System-level failures, patient-related factors, therapy complexity [82] [85] |
| United States | 125,000 | $529 billion (2016 data) | Prescription abandonment, mistrust in healthcare systems, cost barriers [82] [85] |
| Global | Significant proportion of preventable hospitalizations | Billions in avoidable costs across systems | Multi-factorial: patient, provider, and system-level contributors [85] |
Special Challenges in Vitamin D and Nutritional Supplementation
Vitamin D supplementation faces unique adherence challenges across population groups. Parental noncompliance with infant vitamin D supplementation is widespread, sometimes due to parental perception that their infant does not like the supplement [84]. For older adults, adherence to oral nutritional supplements (ONS) is significantly challenged by age-related factors, with a significant negative correlation between ONS adherence and average patient age [86].
Table 2: Key Drivers of Oral Nutritional Supplement (ONS) Non-Adherence in Vulnerable Populations
| Adherence Factor | Impact Level | Research Findings | Target Population |
|---|---|---|---|
| Product Taste & Sensory Properties | High | Studies using flavor variety showed significantly greater compliance (81%) vs. those using variety of ONS types (63%) [86] | All populations, especially those with taste/smell alterations (e.g., cancer patients) |
| Supplement Volume | Medium-High | ONS with >2 kcal/ml energy density had 91% compliance vs. 77% for 1-1.3 kcal/ml formats [86] | Older adults, patients with compromised appetite, cancer patients |
| Age-Related Factors | High | Significant negative correlation between adherence and patient age; older patients face greater challenges [86] | Elderly populations (vulnerable elderly) |
| Taste and Smell Alterations (TSAs) | Medium-High | 20-86% of cancer patients have taste alteration; 5-60% experience smell alterations [86] | Patients undergoing chemotherapy, older adults |
Experimental Protocols for Adherence Research
Protocol: Educational Intervention to Improve Clinician-Patient Supplement Teaching
This protocol adapts a successful quality improvement project that increased vitamin D education for parents of human milk-fed infants by 55% [87], modifying it for vitamin D supplementation in older adults.
3.1.1 Study Design
- Approach: Quasi-experimental, pretest-posttest design
- Duration: 12-week intervention period with 4-week baseline and 4-week follow-up assessment
- Participants: 5-10 clinical providers (physicians, nurse practitioners, physician assistants) and their elderly patients (≥65 years) prescribed vitamin D supplementation
- Setting: Primary care clinics specializing in geriatric care
3.1.2 Intervention Components
- Clinician Education Session: 45-minute structured session covering:
- Electronic Health Record (EHR) Integration:
- Structured vitamin D template embedded within standard documentation workflows
- Includes fields for: supplementation dose, adherence assessment, patient barriers, educational resources provided
- Automated reminders for adherence assessment at follow-up visits
3.1.3 Data Collection and Measures
- Primary Outcome: Number of documented vitamin D teaching episodes
- Secondary Outcomes:
- Clinician knowledge assessed via pre- and post-intervention surveys
- Patient adherence measured through self-report and prescription refill data
- Provider satisfaction with intervention components
- Data Extraction: Chart review for first four consecutive patient visits post-intervention
3.1.4 Analysis Plan
- Descriptive statistics to characterize teaching episodes and adherence
- Paired t-tests to compare pre- and post-intervention measures
- Thematic analysis of provider feedback on intervention utility
Protocol: Multidimensional Analysis of Supplement Adherence Drivers
This protocol provides a framework for investigating the complex factors influencing supplement adherence in older adult populations, based on comprehensive adherence models [83] [82].
3.2.1 Study Design
- Approach: Mixed-methods, combining qualitative and quantitative approaches
- Duration: 6-month recruitment and data collection period
- Participants: 100-150 vulnerable older adults (≥65 years) with prescribed vitamin D supplementation, purposively sampled to include those with multimorbidity, polypharmacy, and socioeconomic challenges
- Setting: Community-dwelling older adults receiving home care services [83]
3.2.2 Data Collection Methods
- Focus Groups: 4-6 homogenous focus groups (6-11 participants each) stratified by:
- Community nurses and home care providers
- Social care service providers
- Volunteers from non-governmental organizations
- Informal caregivers and family members
- Semi-structured Interviews: Individual interviews with vulnerable elderly patients
- Adherence Measures:
- Self-reported adherence using validated scales
- Pill counts for vitamin D supplementation
- Pharmacy refill records
- Contextual Data:
- Medication reviews documenting polypharmacy
- Cognitive and functional assessments
- Health literacy evaluation
3.2.3 Qualitative Analysis
- Inductive Content Analysis:
- Transcription of audio recordings
- Open coding of transcripts by two independent researchers
- Category development through iterative consensus process
- Abstraction to develop comprehensive framework of barriers and facilitators
- Trustworthiness Measures:
- Credibility through exact transcription and independent analysis
- Dependability via detailed documentation of procedures
- Confirmability through ongoing review and refinement
- Transferability through comprehensive context description [83]
3.2.4 Quantitative Analysis
- Descriptive statistics to characterize the study population
- Correlation analysis between adherence rates and identified barriers/facilitators
- Regression models to identify key predictors of non-adherence
Visualization of Adherence Framework
Adherence Barriers and Intervention Framework
This diagram illustrates the multidimensional nature of supplement non-adherence and corresponding evidence-based intervention strategies, synthesized from current research [83] [82] [86]. The framework emphasizes that effective adherence support requires addressing patient, therapy, system, and socioeconomic factors through targeted, multifaceted approaches.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Materials for Adherence Studies
| Research Tool | Function/Application | Protocol Context | Key Considerations |
|---|---|---|---|
| Focus Group Interview Guides | Semi-structured questions to explore barriers and facilitators | Qualitative adherence research [83] | Should be iteratively developed based on literature review and clinical expertise |
| Adherence Survey Instruments | Validated scales for measuring self-reported adherence | Pre-post intervention assessment [87] | Should be complemented by objective measures (e.g., pill counts, refill records) |
| Electronic Health Record (EHR) Templates | Structured documentation tools for supplementation counseling | Educational intervention studies [87] | Must be seamlessly integrated into clinical workflow for maximum adoption |
| Text Mining Algorithms | Natural language processing to classify supplement use status from clinical notes | Retrospective adherence analysis [88] | SVM with n-gram features achieved F-measure of 0.906 for continuing status classification |
| Medication Adherence Scales | Standardized instruments (e.g., Morisky Medication Adherence Scale) | Quantifying adherence levels in study populations | Should be validated for specific population (elderly, low literacy, etc.) |
| Behavioral Change Technique Checklists | Structured inventory of applied behavioral methods | Implementation fidelity assessment [85] | 84% of a:care program HCPs implemented behavioral change techniques vs. 60% in control group |
Addressing patient non-adherence requires a multidimensional approach that recognizes the complex interplay of behavioral, systemic, and therapy-related factors. The protocols and frameworks presented herein provide researchers with validated methodologies to investigate and intervene upon the critical challenge of supplementation adherence. Future research priorities should include greater integration of digital health technologies, implementation of value-based healthcare models that reward adherence outcomes, and development of novel formulation strategies that address sensory and practical barriers to supplementation [82] [86]. By applying these structured approaches, researchers and healthcare systems can meaningfully impact the silent epidemic of non-adherence, ultimately improving patient outcomes and healthcare efficiency.
The determination of when to implement laboratory testing for vitamin D status in older adults represents a significant challenge in clinical practice and research. Widespread supplementation and testing have historically been practiced despite limited evidence supporting broad population-level benefits. Recent clinical practice guidelines have substantially refined this approach, moving from indiscriminate testing toward a targeted screening strategy based on rigorous assessment of evidence from randomized controlled trials. This paradigm shift emphasizes identifying specific at-risk populations who may benefit from intervention, while avoiding unnecessary testing and supplementation in the general older adult population without clear indications. The evolution of these recommendations reflects an increasingly sophisticated understanding of vitamin D metabolism and its complex relationship with health outcomes beyond bone metabolism. This application note provides a comprehensive framework for implementing these evidence-based guidelines within research and clinical development contexts, with particular emphasis on practical methodological considerations for defining and studying at-risk populations.
Current Guideline Recommendations
Major professional societies have reached consensus on restricting routine vitamin D screening for the general older adult population, instead recommending targeted approaches for specific at-risk subgroups. The table below summarizes recommendations from key organizations:
Table 1: Vitamin D Guideline Recommendations for Older Adults
| Organization | Year | General Population Recommendation | At-Risk Population Recommendation | Key Populations Identified |
|---|---|---|---|---|
| Endocrine Society | 2024 | Does not recommend routine testing or supplementation beyond RDA for adults up to 74 years | Recommends supplementation for adults ≥75 years, pregnant women, and people with prediabetes [13] | Older adults (≥75 years), pregnant women, prediabetes, infants/children [13] |
| USPSTF | 2024 (Draft) | Recommends against vitamin D supplementation for primary prevention of fractures and falls in community-dwelling adults ≥60 years [24] | Evidence insufficient for those with established osteoporosis, vitamin D deficiency, or malabsorption [24] | Community-dwelling postmenopausal women and men ≥60 years [24] |
| Systematic Review (Zemp et al.) | 2025 | No guideline recommended screening for the general population; no recommendation for supplementation without risk factors [71] | Two-thirds of guidelines recommended screening for people at risk; half recommended supplementation for at-risk individuals [71] | People with osteoporosis, older adults, conditions increasing deficiency risk [71] |
The Endocrine Society's 2024 guideline update represents the most current comprehensive framework, specifically recommending against routine 25-hydroxyvitamin D [25(OH)D] testing in the general population due to lack of clinical evidence supporting its necessity [13]. Instead, the guidelines advocate for targeted supplementation rather than widespread screening or high-dose supplementation. The society generally endorses the National Academy of Medicine's Recommended Dietary Allowance (600 IU/day for ages up to 70 and 800 IU/day for ages 71 and above) for the general population without additional supplementation unless clinically indicated [13].
The U.S. Preventive Services Task Force (USPSTF) similarly recommends against vitamin D supplementation with or without calcium for the primary prevention of fractures in community-dwelling postmenopausal women and men age 60 years or older, concluding with moderate certainty that supplementation has no net benefit [24]. This recommendation is based on pooled analyses of multiple randomized clinical trials showing no statistically significant difference in hip fracture risk (relative risk 0.99, 95% CI, 0.86 to 1.13), major osteoporotic fracture risk (relative risk 0.93, 95% CI, 0.78 to 1.10), or fall risk [24].
A recent systematic review of clinical guidelines published between 2013 and 2024 confirmed this consensus, finding that no guideline recommended screening or supplementation for the general adult population without risk factors [71]. This analysis of 31 guidelines revealed that while recommendations varied significantly for specific at-risk populations, there was unanimous agreement against population-wide screening approaches.
Defining At-Risk Populations and Research Considerations
Established At-Risk Populations
Current evidence supports identifying several specific populations who may benefit from targeted vitamin D status assessment and supplementation:
Adults aged 75 years and older: The Endocrine Society specifically recommends supplementation for this group due to decreased skin synthesis of vitamin D and reduced intestinal absorption associated with aging [13]. The potential benefits include reduced risk of mortality based on randomized controlled trial evidence [13].
Individuals with prediabetes: Supplementation in this population may help reduce disease progression to type 2 diabetes, representing a significant preventive intervention given that approximately one in three adults in the United States has prediabetes [13].
People with conditions affecting vitamin D metabolism: This includes those with malabsorption syndromes, hepatic or renal impairment, and specific bone metabolism disorders, though the USPSTF notes insufficient evidence for these subgroups in their general recommendations [24].
Osteoporosis patients: The systematic review by Zemp et al. found that one-third of guidelines targeted people with osteoporosis, recommending vitamin D supplementation with varying doses, mainly between 400 to 1000 IU/day [71].
Institutionalized or hospitalized older adults: While not explicitly covered in the general guidelines, these populations typically have significantly different risk profiles than community-dwelling adults and may require distinct approaches.
Critical Research Gaps
Despite these clear guidelines, significant research gaps remain that merit investigation:
The optimal dosing regimens for different at-risk populations require clarification through randomized controlled trials specifically designed with these subgroups as the target population [71].
The impact of genetic polymorphisms in the vitamin D receptor on treatment efficacy and metabolism needs further characterization [13].
Long-term safety data beyond hypercalcemia risks are insufficient, particularly for high-dose supplementation regimens [13].
Research across diverse racial, ethnic, and geographic populations is needed, as most current evidence comes from high-income, developed nations [13].
The role of vitamin D in non-skeletal outcomes including cardiovascular disease, autoimmune diseases, and cancer prevention requires further investigation through well-designed clinical trials [13].
Methodological Protocols for Vitamin D Research
Laboratory Assessment Protocol
Sample Collection and Processing:
- Collect serum samples in sterile vacuum containers without anticoagulants
- Allow samples to clot completely at room temperature for 30-45 minutes
- Centrifuge at 2000-3000 × g for 15 minutes at 4°C
- Aliquot serum into cryovials and store at -80°C until analysis
- Avoid repeated freeze-thaw cycles (maximum 2-3 cycles recommended)
25-Hydroxyvitamin D Analysis:
- Methodology: Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the gold standard method for 25(OH)D quantification
- Quality Control: Implement three levels of quality control materials with each analytical run
- Standardization: Ensure methodology is traceable to standard reference materials (NIST SRM 972a)
- Reporting: Express concentrations in nmol/L (or ng/mL with conversion factor: 1 ng/mL = 2.5 nmol/L)
Table 2: Research Reagent Solutions for Vitamin D Assessment
| Reagent/Material | Specifications | Application/Function |
|---|---|---|
| 25(OH)D₂/D₃ Standards | Certified reference materials, purity >97% | Calibration curve preparation for LC-MS/MS quantification |
| Deuterated Internal Standards | 25(OH)D₃-d₆, 25(OH)D₂-d₃ | Correct for matrix effects and recovery variations in MS methods |
| Solid Phase Extraction Plates | C18 bonded silica, 96-well format | Sample cleanup and analyte enrichment prior to analysis |
| LC-MS/MS Mobile Phase | Methanol with 0.1% formic acid; Water with 0.1% formic acid | Chromatographic separation of vitamin D metabolites |
| Quality Control Materials | Three levels (low, medium, high) covering clinical range | Method validation and continuous quality assurance |
| Protein Precipitation Reagent | Acetonitrile with internal standard | Serum protein removal in sample preparation |
| Derivatization Reagents | PTAD (4-phenyl-1,2,4-triazoline-3,5-dione) | Enhancement of detection sensitivity in mass spectrometry |
Clinical Trial Design Considerations
For interventional studies investigating vitamin D efficacy:
- Population Stratification: Pre-specify subgroup analyses based on baseline 25(OH)D levels, age categories, BMI, and comorbidities
- Dosing Regimens: Consider both daily and intermittent (weekly, monthly) dosing protocols with appropriate pharmacokinetic assessments
- Outcome Measures: Include both primary (disease-specific) and secondary (intermediate) endpoints with adequate powering for each
- Adherence Assessment: Implement pill counts, serum 25(OH)D measurements, and digital monitoring when possible
- Safety Monitoring: Regular assessment of serum calcium, creatinine, and adverse event reporting
The following workflow diagram illustrates the decision-making process for vitamin D testing and supplementation in older adult populations:
The contemporary approach to vitamin D testing in older adults has evolved toward a precision medicine model that emphasizes selective screening and targeted supplementation based on individual risk factors rather than population-wide interventions. Researchers and clinicians should prioritize identification of established at-risk populations—particularly adults ≥75 years, those with prediabetes, and individuals with conditions affecting vitamin D metabolism—for focused assessment and intervention. The methodological frameworks provided in this application note offer standardized protocols for implementing these guidelines in research settings while highlighting critical evidence gaps that warrant further investigation. As new evidence emerges from ongoing clinical trials, these recommendations will continue to refine our understanding of optimal vitamin D management across the heterogeneity of the older adult population.
Vitamin D is an essential regulator of calcium and phosphate homeostasis, playing a critical role in bone health and numerous physiological processes [89]. While severe vitamin D deficiency leads to well-established clinical conditions like rickets in children and osteomalacia in adults, the optimal vitamin D status for older adults—and the point at which supplementation becomes harmful—remains a nuanced clinical challenge [89] [90]. The therapeutic window for vitamin D supplementation appears to be narrower than previously recognized, with safety considerations that depend not only on dosage but also on treatment regimen, age, sex, and baseline vitamin D status [89]. This application note examines the critical differentiation between achieving vitamin D sufficiency and risking hypervitaminosis D, with specific focus on implications for older adult research and therapeutic development.
Quantitative Analysis: Vitamin D Dosing, Efficacy, and Safety Thresholds
Vitamin D Dosing Ranges and Associated Outcomes
Table 1: Vitamin D Supplementation Doses and Documented Effects in Older Adults
| Daily Dose Equivalent | Serum 25(OH)D Level | Efficacy Outcomes | Safety Risks/Adverse Events |
|---|---|---|---|
| 400-800 IU [89] | ~50 nmol/L [89] | Prevents nutritional rickets and osteomalacia; insufficient for fracture risk reduction [89] | No hypercalcemia reported [89] |
| 800-1000 IU [89] | ≥50 nmol/L [89] | Consistent recommendation for oldest old; reduces hip fracture risk when combined with calcium [89] | Considered safe for prevention/correction of deficiency [89] |
| 2000-4000 IU [52] | ~100-120 nmol/L [89] | Non-significant trend in slowing progression from prediabetes to T2DM [90] | 3% hypercalcemia incidence (4000 IU); increased hypercalciuria [89] |
| 10,000 IU [89] | >125 nmol/L [89] | Associated with accelerated radial bone loss (−3.5% vBMD over 3 years) [89] | 9% hypercalcemia incidence; 31% hypercalciuria incidence [89] |
Clinical Guideline Recommendations for Different Populations
Table 2: Summary of Vitamin D Recommendations Across Guidelines for Various Populations
| Population | Screening Recommendation | Supplementation Recommendation | Target Serum 25(OH)D |
|---|---|---|---|
| General Adult Population | Not recommended [18] | Not recommended without risk factors [18] | N/A |
| Older Adults (≥60-70 years) | Recommended for those at risk [18] | 600-800 IU/day [52]; 800-1000 IU/day for fracture prevention [89] | 50 nmol/L (20 ng/mL) [89] |
| Adults with Osteoporosis | Recommended [18] | 400-1000 IU/day (guidelines vary) [18] | 50-75 nmol/L [18] |
| Adults with Risk Factors* | Recommended [18] | Varies by condition; generally recommended [18] | Varies by condition |
*Risk factors include chronic kidney disease, malabsorption syndromes, obesity, medications affecting vitamin D metabolism, and low sun exposure [18].
Pathophysiological Mechanisms: From Sufficiency to Toxicity
Vitamin D Metabolism and Toxicity Pathways
Diagram 1: Vitamin D metabolic pathway and toxicity mechanisms. Excessive vitamin D leads to hypercalcemia through increased intestinal calcium absorption and bone resorption.
Vitamin D toxicity manifests primarily through disruption of calcium homeostasis. The active metabolite 1,25-dihydroxyvitamin D (calcitriol) enhances intestinal trans-epithelial calcium transport through both genomic and non-genomic mechanisms [89]. Simultaneously, calcitriol acts as a potent stimulator of bone resorption by increasing RANKL expression in osteoblasts [89]. When these processes become excessive due to supraphysiological vitamin D levels, the resulting hypercalcemia can lead to clinical symptoms including nephrolithiasis, renal impairment, neuromuscular dysfunction, and soft tissue calcification [89] [52].
The half-life of 25-hydroxyvitamin D (25OHD) is approximately 2-3 weeks, meaning that hypercalcemic-hypercalciuric syndrome can persist for several weeks to months after discontinuation of high-dose supplementation, with potential for extensive and permanent soft tissue damage from mineral deposits [89].
Research Methodologies: Assessing Efficacy and Safety Endpoints
Experimental Protocol for Vitamin D Supplementation Trials
Objective: To evaluate the efficacy and safety of vitamin D supplementation in older adults (≥60 years) with vitamin D insufficiency.
Inclusion Criteria:
- Community-dwelling adults aged ≥60 years
- Serum 25(OH)D levels 25-50 nmol/L
- No history of osteoporotic fractures, kidney stones, or conditions affecting vitamin D metabolism
Exclusion Criteria:
- Renal impairment (eGFR <30 mL/min/1.73m²)
- Hypercalcemia (serum calcium >2.55 mmol/L)
- Use of thiazide diuretics, anticonvulsants, or other medications that interfere with vitamin D metabolism
Study Design:
- Randomized, double-blind, placebo-controlled trial
- 3-arm design: 400 IU/d (control), 2000 IU/d, 4000 IU/d
- Duration: 36 months
Primary Efficacy Endpoints:
- Change in areal BMD at hip and spine (DXA)
- Incident fragility fractures (vertebral, non-vertebral, hip)
- Falls incidence (prospective monthly calendars)
Primary Safety Endpoints:
- Serum calcium (monthly for 3 months, then quarterly)
- 24-hour urinary calcium excretion (baseline, 12, 24, 36 months)
- Renal function (eGFR, serum creatinine)
- Incident kidney stones
Statistical Analysis:
- Intention-to-treat analysis
- Pre-specified subgroup analysis by baseline 25(OH)D, BMI, age
Table 3: Schedule of Assessments for Vitamin D Supplementation Trial
| Assessment | Baseline | 3 Months | 6 Months | 12 Months | 24 Months | 36 Months |
|---|---|---|---|---|---|---|
| Medical History | X | |||||
| Physical Exam | X | X | X | |||
| Weight/BMI | X | X | X | X | X | X |
| Serum 25(OH)D | X | X | X | X | X | X |
| Serum Calcium | X | X | X | X | X | X |
| Serum Creatinine | X | X | X | X | X | X |
| 24-h Urine Calcium | X | X | X | |||
| DXA BMD | X | X | X | |||
| Falls Assessment | X (retro) | Monthly | Monthly | Monthly | Monthly | Monthly |
| Fracture Adjudication | X | X | X | X | X | X |
| Adverse Events | X | X | X | X | X | X |
Decision Framework for Vitamin D Supplementation in Older Adults
Diagram 2: Clinical decision pathway for vitamin D management in older adults, emphasizing safety monitoring.
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Table 4: Key Research Reagent Solutions for Vitamin D Investigations
| Reagent/Material | Specifications | Research Application |
|---|---|---|
| 25-Hydroxyvitamin D Standardized Assay | LC-MS/MS preferred for accuracy | Gold standard for assessing vitamin D status; essential for baseline and follow-up measurements [89] |
| Cholecalciferol (Vitamin D3) | >98% purity; stability-tested | Most common supplementation form; preferred over ergocalciferol (D2) for longer half-life [24] [52] |
| Calcifediol (25OHD) | Pharmaceutical grade | Research alternative to cholecalciferol; ~3x more potent; useful in malabsorption studies [89] |
| Calcium Assay Kit | Colorimetric/atomic absorption | Critical safety parameter; hypercalcemia primary indicator of toxicity [89] [52] |
| Parathyroid Hormone (PTH) ELISA | Intact PTH detection | Assess physiological response to vitamin D status; inverse relationship with 25(OH)D [89] |
| Bone Turnover Markers | CTX (resorption), P1NP (formation) | Monitor bone remodeling effects of vitamin D supplementation [89] |
| VDR Antibodies | Validated for IHC/Western blot | Study vitamin D receptor expression in target tissues [90] |
| CYP27B1 (1α-hydroxylase) Assay | Activity measurement or gene expression | Evaluate activation of vitamin D to calcitriol in renal and extra-renal tissues [90] |
Current evidence indicates that vitamin D supplementation provides the greatest benefit when targeted to individuals with documented deficiency, particularly for skeletal outcomes [90] [18]. The established upper limit of 4000 IU/day requires careful consideration in older adults, as emerging data suggest potential adverse effects on bone health at doses exceeding 4000 IU/day, even without overt hypercalcemia [89]. Research protocols should prioritize systematic safety monitoring that includes serial measurements of serum and urinary calcium, particularly when implementing higher-dose regimens. Future clinical trials would benefit from designs that incorporate achieved 25(OH)D concentrations rather than solely relying on intention-to-treat analyses, enabling more precise determination of optimal therapeutic ranges for different older adult populations while maintaining appropriate safety margins [91].
Critical Appraisal of Guideline Consensus, Disparities, and Evidence Quality
This systematic review synthesizes and compares recent clinical practice guidelines from major endocrine and public health institutions regarding vitamin D supplementation and screening. The analysis focuses on the 2024 Endocrine Society Clinical Practice Guideline, frameworks from the Institutes of Medicine (IOM), and recommendations from the UK's National Health Service (NHS), with particular emphasis on applications for older adult populations. Findings reveal a emerging consensus against routine population-wide vitamin D testing and a more targeted approach to supplementation. Key populations identified for empiric supplementation include adults over 75 years, individuals with prediabetes, pregnant people, and children. Significant variation persists in specific dosage recommendations and optimal serum 25-hydroxyvitamin D thresholds, highlighting critical knowledge gaps for future research.
Vitamin D management represents a significant challenge in public health and clinical practice, with numerous studies demonstrating associations between serum 25-hydroxyvitamin D (25[OH]D) concentrations and musculoskeletal, metabolic, cardiovascular, malignant, autoimmune, and infectious diseases [92]. Despite these associations, causal relationships remain difficult to establish, leading to substantial variation in clinical guidelines. This systematic review analyzes major guidelines current through 2024-2025, focusing specifically on their implications for older adult research and clinical practice. The escalating rates of vitamin D supplementation and testing, coupled with increasing healthcare costs, underscore the urgency of establishing evidence-based consensus recommendations [41] [93].
Table 1: Vitamin D Supplementation Recommendations by Population Group
| Population Group | Endocrine Society (2024) [12] [92] | IOM/DRI Recommendations [94] | NHS England (2025) [93] [95] |
|---|---|---|---|
| Children & Adolescents (1-18 years) | Empiric supplementation recommended to prevent rickets and lower respiratory infection risk | 600 IU/day (Ages 1-70) [94] | 400 IU/day for children aged 4+; all exclusively/partly breastfed infants until age 1 year [93] |
| Adults (19-49 years) | Against supplementation beyond RDI for healthy adults | 600 IU/day [94] | Supplementation only for at-risk groups [93] |
| Adults (50-74 years) | Against supplementation beyond RDI for healthy adults | 600 IU/day [94] | Supplementation only for at-risk groups [93] |
| Older Adults (75+ years) | Empiric supplementation recommended to lower mortality risk | 800 IU/day (Ages 70+) [94] | Supplementation recommended for at-risk older adults [93] |
| Pregnancy | Empiric supplementation recommended to reduce risk of preeclampsia, preterm birth, and neonatal mortality | 600 IU/day [94] | Supplementation for pregnant people at risk of deficiency [93] |
| Prediabetes | Empiric supplementation recommended to reduce progression to diabetes | Not specified | Not specified |
| Dosing Preference | Daily, lower-dose vitamin D over non-daily, higher doses for adults ≥50 years | Not specified | Colecalciferol (vitamin D3) preferred [93] |
Table 2: Vitamin D Testing and Threshold Recommendations
| Parameter | Endocrine Society (2024) [12] [92] | IOM Framework | NHS England (2025) [93] [95] |
|---|---|---|---|
| Routine Screening | Recommends against routine 25(OH)D testing in healthy populations, including those with obesity or dark complexion | Not specified | Testing not recommended in most cases; empiric supplementation based on risk [93] |
| Testing Indications | Not recommended in absence of established indications | Not specified | Appropriate for investigating possible rickets or osteomalacia; consider for pregnant people with multiple risk factors [93] |
| Deficiency Threshold | No specific levels defined due to insufficient evidence | Not specified | < 25 nmol/L [95] |
| Insufficiency Threshold | No specific levels defined due to insufficient evidence | Not specified | 25-50 nmol/L [95] |
| Optimal Level | No specific target levels defined for disease prevention | Not specified | ≥ 75 nmol/L [95] |
| At-Risk Populations Identified | Older adults (>75), pregnant people, children, prediabetes | Not specified | Naturally dark skin, minimal sun exposure, southern regions in winter, aged care residents, reduced mobility [93] |
Experimental Protocols for Guideline Development
Systematic Review Methodology for Guideline Development
The 2024 Endocrine Society guideline employed rigorous systematic review methodology that serves as a template for evidence-based guideline development [41] [92]:
Literature Search Strategy:
- Comprehensive electronic database searches (PubMed, Embase, Cochrane Library)
- Search timeframe: January 2000 through June 2024
- Priority given to randomized placebo-controlled trials in general populations without established indications for vitamin D treatment
- Focus on empiric vitamin D administration throughout lifespan and in select conditions (pregnancy, prediabetes)
Inclusion/Exclusion Criteria:
- Included: Clinical guidelines, systematic reviews, RCTs, observational studies with clear methodologies
- Excluded: Editorials, conference abstracts, narrative reviews, studies limited to institutionalized populations
- Restricted to publications in English from Europe or North America
Evidence Assessment:
- Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology to assess certainty of evidence
- Multidisciplinary panel of clinical experts and methodology experts
- Consideration of patient values, costs, resources, acceptability, feasibility, and health equity impacts
Delphi Consensus Protocol for Guideline Development
The Egyptian Academy for Bone and Muscle Health guideline development employed a formal Delphi process that represents best practices for achieving expert consensus [96]:
Expert Panel Formation:
- 11 experts selected based on recognized expertise in vitamin D research and clinical management
- Multidisciplinary representation including rheumatology, endocrinology, clinical pathology, nutritional medicine
Consensus Process:
- Two iterative rounds of anonymous questionnaires
- Statements rated on 1-9 scale (1=do not agree, 9=strongly agree)
- Agreement threshold set at >80% for final recommendations
- Opportunity for statement modification between rounds based on aggregated feedback
Outcome Measurement:
- Consensus stability assessment through multiple rounds
- Documentation of modified statements and rationale for changes
- Final approval of all statements exceeding the 80% agreement threshold
Visualization of Guideline Development and Implementation Pathways
Vitamin D Guideline Development Methodology
Figure 1: Guideline Development Workflow. This diagram illustrates the evidence-based methodology for clinical practice guideline development, incorporating systematic review and formal consensus processes.
Vitamin D Clinical Decision Pathway for Older Adults
Figure 2: Older Adult Clinical Decision Pathway. This algorithm outlines the vitamin D management approach for older adults based on current guideline recommendations, emphasizing empiric supplementation for those ≥75 years.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Materials for Vitamin D Clinical Studies
| Research Reagent | Function/Application in Vitamin D Research |
|---|---|
| 25-Hydroxyvitamin D Assays | Gold standard for assessing vitamin D status; used to measure circulating 25(OH)D levels in clinical trial participants [92] |
| Cholecalciferol (Vitamin D3) | Native form of vitamin D used in majority of clinical trials; preferred for supplementation studies due to superior bioavailability compared to ergocalciferol [93] |
| Ergocalciferol (Vitamin D2) | Plant-derived vitamin D form; used in comparative studies evaluating efficacy differences between D2 and D3 formulations [96] |
| Calcitriol (1,25-Dihydroxyvitamin D) | Active vitamin D metabolite; used in mechanistic studies to understand vitamin D receptor signaling and molecular pathways [96] |
| Vitamin D Receptor Antibodies | Essential for immunohistochemistry and Western blot analyses to study tissue-specific vitamin D receptor expression and distribution [96] |
| PARATHYRosine Assays | Used to measure parathyroid hormone levels; critical for assessing secondary hyperparathyroidism as functional indicator of vitamin D deficiency [93] |
| Calcium and Phosphate Kits | For measuring serum calcium and phosphate levels; essential parameters in bone metabolism studies related to vitamin D status [93] |
| FGF-23 ELISA Kits | For measuring fibroblast growth factor 23 levels; important for studies investigating vitamin D metabolism and phosphate regulation [96] |
Discussion and Research Implications
The 2024 guideline analysis reveals a significant evolution in vitamin D recommendations, with important implications for older adult research. The shift toward targeted supplementation in specific risk groups (particularly adults over 75 years) rather than population-wide approaches reflects growing evidence from randomized controlled trials. The consistent recommendation against routine vitamin D testing across guidelines highlights the limited utility of widespread screening and emphasizes the cost-effectiveness of empiric supplementation in identified risk groups.
The persistence of several knowledge gaps presents critical opportunities for future research. The inability to define specific 25(OH)D target levels for disease prevention, optimal dosing regimens for different populations, and the molecular mechanisms underlying vitamin D's potential extra-skeletal effects represent key areas requiring investigation. For the older adult population specifically, research should focus on determining whether vitamin D supplementation in those over 75 years provides benefits beyond mortality reduction, including effects on musculoskeletal health, immune function, and chronic disease prevention.
The methodological approaches outlined in this analysis, particularly the rigorous systematic review and Delphi consensus processes, provide templates for developing evidence-based guidelines in nutritional research. Future guideline development would benefit from standardized outcomes assessment, harmonized dosing protocols, and inclusion of diverse populations to ensure broad applicability of recommendations.
The role of vitamin D in disease prevention, particularly for older adults, has been a subject of intense research and debate. Over the past decade, clinical guidelines have evolved significantly as new evidence from large-scale trials has emerged. Guideline development panels have faced the challenge of interpreting sometimes conflicting study results to form practical recommendations for clinicians and researchers [10] [94]. This application note systematically compares contemporary vitamin D guidelines, with a specific focus on their implications for older adult research and the methodological considerations for studying this population. The Endocrine Society's 2024 guideline update represents a significant shift from their 2011 positions, particularly regarding population-wide screening and supplementation in generally healthy individuals [10] [12]. These changes reflect insights from recent large trials but also highlight enduring evidence gaps that remain ripe for scientific investigation.
Comparative Analysis of Major Guidelines
Table 1: Comparison of Vitamin D Guideline Recommendations for Older Adults
| Guideline Aspect | Endocrine Society (2011) | Endocrine Society (2024) | Systematic Review of Guidelines (2025) |
|---|---|---|---|
| Target 25(OH)D Level | 30 ng/mL (sufficiency) [94] | No specific target level established [94] | Varying thresholds (50-75 nmol/L or 20-30 ng/mL) across guidelines [41] |
| Screening Recommendation | Implied for at-risk groups [94] | Against routine testing in healthy populations [12] | No consensus; some recommend for at-risk groups only [41] |
| Daily RDI for >70 years | 800 IU [94] | 800 IU (maintains IOM RDI) [94] | 400-1000 IU (varied by guideline) [41] |
| Empiric Supplementation >75 years | Not specifically addressed | Recommended for mortality risk reduction [12] [94] | Recommended in some guidelines for older populations [41] |
| Dosing Frequency Preference | Daily or weekly considered appropriate [94] | Prefer daily, lower-dose over non-daily, higher-dose for >50 [12] [94] | Not consistently addressed across guidelines [41] |
Analysis of Key Divergences
The comparative analysis reveals several critical divergences in vitamin D recommendations with significant implications for research design:
Target Level Controversy: The most striking divergence concerns optimal 25-hydroxyvitamin D (25(OH)D) levels. The 2011 Endocrine Society guideline established a 30 ng/mL sufficiency threshold [94], while the 2024 update explicitly does not endorse specific target levels, citing insufficient evidence to determine "blood-level thresholds for 25-hydroxyvitamin D for adequacy or for target levels for disease prevention" [12]. This reflects ongoing debate in the scientific community, with some experts suggesting the current 20 ng/mL cutoff may be too high, potentially overestimating deficiency prevalence [97].
Screening Philosophy: Guideline organizations consistently recommend against population-wide screening [41] [12], with the 2024 Endocrine Society guideline explicitly suggesting "against routine testing for 25-hydroxyvitamin D levels in any of the populations studied" [12]. This represents a significant shift from earlier practices where screening was more commonplace.
Population-Specific Recommendations: The 2024 guidelines introduce nuanced recommendations for specific populations. Adults over 75 years now receive a specific recommendation for empiric supplementation beyond the RDI for potential mortality risk reduction, whereas the 2011 guidelines provided more generalized age-based dosing [94]. This reflects emerging evidence from trials focusing on older adult cohorts [12].
Experimental Protocols for Vitamin D Research in Aging
Dosing Interval Efficacy Protocol
A critical methodological consideration for vitamin D research involves dosing schedule efficacy. The following protocol is adapted from Chel et al.'s randomized controlled trial that directly compared equivalent vitamin D3 doses administered at different intervals in elderly nursing home residents [56] [98]:
- Study Population: Recruit older adults (e.g., ≥70 years) with confirmed vitamin D deficiency (25(OH)D <50 nmol/L). The Chel et al. study specifically enrolled nursing home residents (mean age 84±6.3 years) with limited sun exposure [56].
- Randomization: Assign participants to one of three intervention groups:
- Daily dosing: 600 IU vitamin D3
- Weekly dosing: 4,200 IU vitamin D3 (equivalent to 600 IU/day)
- Monthly dosing: 18,000 IU vitamin D3 (equivalent to 600 IU/day)
- Control: Include placebo group for comparison [56]
- Duration: 4 months intervention period
- Primary Outcome: Serum 25(OH)D levels at baseline and 4 months
- Secondary Outcomes: Parathyroid hormone (PTH) levels, bone turnover markers (e.g., CTX), safety parameters (serum calcium, renal function) [56] [98]
- Key Findings Reference: In the original study, daily dosing proved most effective (69.9 nmol/L), followed by weekly (67.2 nmol/L), with monthly dosing least effective (53.1 nmol/L) in raising 25(OH)D levels [56].
Advanced Biomarker Assessment Protocol
Contemporary vitamin D research increasingly incorporates sophisticated biomarkers of aging. The following protocol adapts methodology from the DO-HEALTH trial analysis examining biological aging measures [8]:
- Study Population: Generally healthy older adults (≥70 years). The DO-HEALTH Bio-Age sub-study included 777 participants with mean age 75 years [8].
- Intervention Design: 2×2×2 factorial design testing:
- Vitamin D3 (2,000 IU/day)
- Omega-3 (1 g/day)
- Simple Home Exercise Program (SHEP; 3×30 min/week)
- Duration: 3-year intervention with annual assessments
- Biological Aging Measures:
- DNA Collection: Blood collection at baseline and 3 years for DNA extraction and biobanking [8]
- DNA Methylation Analysis: Assessment of multiple epigenetic clocks:
- Second-generation clocks: PhenoAge, GrimAge, GrimAge2
- Third-generation clock: DunedinPACE
- First-generation clocks: Horvath, Hannum (for comparison)
- Statistical Analysis: Analysis of covariance comparing change in standardized age acceleration residuals between treatment and control groups, adjusted for chronological age, sex, BMI, and study site [8]
Research Workflow and Signaling Pathways
Vitamin D Research Implementation Workflow
The following diagram illustrates the key decision points and methodological considerations for designing vitamin D clinical trials based on current guideline recommendations and recent research findings:
Research Implementation Workflow
Vitamin D Molecular Signaling Pathway
The following diagram illustrates key molecular pathways through which vitamin D supplementation may influence health outcomes in older adults, based on current mechanistic research:
Molecular Signaling Pathways
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Research Materials for Vitamin D Aging Studies
| Reagent/Material | Specification/Function | Research Application |
|---|---|---|
| Vitamin D3 (Cholecalciferol) | Pharmaceutical grade; various dosing formulations (tablets, capsules, oil solutions) | Intervention substance; requires stability testing for long-term trials [56] [8] |
| 25-Hydroxyvitamin D Assays | CDC-certified methods meeting ±5% mean bias performance criteria [99] | Primary status assessment; essential for standardized measurement across sites [99] |
| DNA Methylation Kits | Bisulfite conversion kits; array-based or sequencing platforms | Epigenetic clock analysis (PhenoAge, GrimAge, DunedinPACE) [8] |
| Parathyroid Hormone (PTH) Immunoassays | Validated for elderly populations with potential renal impairment | Secondary endpoint for bone metabolism [56] [98] |
| Bone Turnover Markers | Serum CTX (resorption), P1NP (formation) markers | Bone-specific endpoint assessment [56] |
| Omega-3 Formulations | High-purity EPA/DHA concentrates (1g/day used in DO-HEALTH) | Combination intervention studies [8] |
The divergent recommendations in vitamin D guidelines highlight both the progress and persistent challenges in nutritional intervention research for older adults. The evolution from the 2011 to 2024 Endocrine Society guidelines demonstrates how accumulating evidence shapes clinical advice, particularly regarding dosing frequency preferences and target population identification. For researchers, these divergences represent opportunities to address critical evidence gaps through methodologically rigorous studies.
Future research should prioritize several key areas: establishing optimal dosing intervals through direct comparative studies, validating epigenetic aging biomarkers as responsive endpoints in vitamin D trials, and clarifying the role of combination interventions (e.g., vitamin D with omega-3 and exercise) for healthy aging. The consistent recommendation against population-wide screening underscores the need for better risk stratification tools to identify older adults most likely to benefit from intervention. By addressing these research priorities while adhering to rigorous methodology standards, scientists can contribute to the next generation of evidence-based vitamin D recommendations tailored to our aging global population.
The development of clinical guidelines for vitamin D supplementation in older adults presents a paradigm for understanding how high-certainty evidence from Randomized Controlled Trials (RCTs) translates into conditional recommendations. Recent years have witnessed significant evolution in this evidence landscape, with large-scale RCTs and meta-analyses challenging long-held assumptions about vitamin D's efficacy for fall and fracture prevention in community-dwelling older adults. This assessment examines the strength of evidence supporting current vitamin D supplementation guidelines, analyzes key methodological approaches in major studies, and provides protocols for evidence synthesis in this contentious research area.
The evidence structure for vitamin D recommendations spans multiple hierarchical levels, from foundational study designs to clinical implementation, as visualized below:
Quantitative Evidence Synthesis: Key Findings from Recent Studies
Table 1: Efficacy of Vitamin D Supplementation on Fall Risk in Older Adults (≥65 Years)
| Study/Type | Participants | Intervention | Comparison | Outcome Measure | Effect Size (95% CI) |
|---|---|---|---|---|---|
| 2025 Meta-Analysis [62] | 23,211 from 10 RCTs | Vitamin D (various doses) | Placebo/no treatment | Fall Risk | OR = 0.99 (0.95-1.03) |
| Subgroup: Women [62] | 13,510 women | Vitamin D (various doses) | Placebo | Fall Risk | OR = 0.97 (0.92-1.02) |
| Subgroup: Men [62] | 9,701 men | Vitamin D (various doses) | Placebo | Fall Risk | OR = 1.08 (0.98-1.20) |
| Subgroup: ≤1000 IU/day [62] | 10,442 participants | ≤1000 IU/day Vitamin D | Placebo | Fall Risk | OR = 0.96 (0.90-1.02) |
| 2024 Network Meta-Analysis [45] | 58,937 from 35 RCTs | 800-1000 IU/day Vitamin D | Placebo/no treatment | Fall Risk | RR = 0.85 (0.74-0.95) |
| Subgroup: Daily Dosing [45] | 22,158 participants | 800-1000 IU/day (daily) | Placebo | Fall Risk | RR = 0.78 (0.64-0.92) |
| Subgroup: Vitamin D Deficient [45] | 8,742 participants | 800-1000 IU/day (≤50 nmol/L) | Placebo | Fall Risk | RR = 0.69 (0.52-0.86) |
Table 2: Major Clinical Guideline Recommendations for Vitamin D in Older Adults (2024-2025)
| Guideline Organization | Population | Recommended Daily Dose | Recommendation Strength | Key Considerations |
|---|---|---|---|---|
| Endocrine Society [10] [100] [92] | Adults ≥75 years | No specific dose (empiric supplementation) | Conditional for ≥75 years | Potential mortality benefit; against routine testing |
| Endocrine Society [10] [92] | Adults 50-74 years | Against supplementation above DRI | Conditional against | No demonstrated benefit in vitamin D-replete adults |
| U.S. National Academy of Medicine [100] | Adults ≥70 years | 800 IU/day | Recommended Dietary Allowance | Based on bone health outcomes |
| Various Guidelines Synthesis [41] | Older adults with osteoporosis | 400-1000 IU/day | Varying strength | Based on individual risk assessment |
| Egyptian Academy [96] | At-risk populations | Individualized dosing | Strong for deficiency | Maintenance >30 ng/mL 25(OH)D |
Experimental Protocols for Vitamin D Research
Protocol for Systematic Review and Meta-Analysis of Vitamin D RCTs
Objective: To synthesize evidence from RCTs evaluating vitamin D supplementation for fall prevention in older adults.
Eligibility Criteria:
- Population: Community-dwelling adults aged ≥65 years (consistent with recent meta-analyses) [62]
- Intervention: Vitamin D2 or D3 supplementation (any dose, frequency, or duration)
- Comparator: Placebo or no treatment
- Outcomes: Fall incidence, fractures, adverse events
- Study Design: RCTs with minimum 3-month follow-up
Search Strategy:
- Database searching: MEDLINE, EMBASE, Cochrane Central (from January 2005 to present)
- Search terms: "vitamin D", "cholecalciferol", "ergocalciferol", "accidental falls", "aged", "randomized controlled trial"
- Language restriction: English, Spanish (based on recent methodology) [62]
- Additional sources: Clinical trial registries, reference lists of included studies
Data Extraction Protocol:
- Use standardized extraction form in Rayyan application or Covidence
- Extract: Participant characteristics, intervention details (dose, frequency, duration), comparator, outcome definitions, results data
- Risk of bias assessment: Cochrane RoB 2.0 tool [62]
- Certainty assessment: GRADE methodology [92]
Statistical Analysis Plan:
- Meta-analysis using inverse variance method
- Random-effects model if I² > 50%
- Dichotomous outcomes: Pooled odds ratios or risk ratios with 95% CI
- Continuous outcomes: Mean differences or standardized mean differences
- Subgroup analyses: Dose, frequency, baseline 25(OH)D levels, gender
- Sensitivity analyses: Leave-one-out, risk of bias stratification
Protocol for Network Meta-Analysis of Different Vitamin D Regimens
Objective: To compare the efficacy of different vitamin D dosing regimens for fall prevention using direct and indirect evidence.
Intervention Nodes:
- Placebo/no treatment
- Vitamin D ≤500 IU/day
- Vitamin D 600-700 IU/day
- Vitamin D 800-1000 IU/day [45]
- Vitamin D 1100-1900 IU/day
- Vitamin D ≥2000 IU/day
- Vitamin D with calcium supplementation
Analysis Methods:
- Bayesian framework using Markov Chain Monte Carlo methods [45]
- Consistency model checking: Deviation Information Criterion (DIC) difference <5 indicates consistency [45]
- Probability ranking: Surface under the cumulative ranking curve (SUCRA) values
- Model implementation: GeMTC and JAGS in R4.1.3 [45]
The network meta-analysis methodology enables comparative effectiveness research across multiple intervention strategies, as illustrated in the following workflow:
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents and Materials for Vitamin D Clinical Research
| Reagent/Material | Specification | Research Application | Key Considerations |
|---|---|---|---|
| Cholecalciferol (Vitamin D3) | Pharmaceutical grade | Gold-standard intervention | Preferred over ergocalciferol in most recent RCTs [62] |
| 25-Hydroxyvitamin D Assay | LC-MS/MS preferred | Status assessment | Gold-standard for measuring 25(OH)D concentrations [90] |
| Placebo | Matching appearance, taste | Control group | Olive oil or medium-chain triglycerides as carriers |
| Calcium Supplements | Carbonate or citrate | Combination therapy | Dosed at 500-1200 mg/day in combination studies [101] |
| Electronic Fall Diaries | Validated self-report | Outcome assessment | Monthly fall calendars with postcard or electronic reporting [62] |
| Bone Turnover Markers | P1NP, CTX | Secondary endpoints | Assess bone remodeling response to supplementation |
| Muscle Function Tests | SPPB, grip strength | Secondary endpoints | Short Physical Performance Battery for functional assessment |
Evidence to Recommendation Framework
Factors Influencing Guideline Decisions
The transformation of evidence into recommendations involves weighing multiple factors beyond efficacy alone:
Balance of Benefits and Harms: Recent meta-analyses demonstrate null effects on fall risk (OR 0.99, 95% CI 0.95-1.03) [62], shifting the risk-benefit calculus toward more conservative recommendations.
Dose-Response Considerations: Evidence suggests a U-shaped relationship, with 800-1000 IU/day potentially beneficial [45] but higher doses (>1000 IU/day) showing no additional benefit and possible harm [62] [45].
Certainty of Evidence: The GRADE approach typically rates vitamin D evidence as low to moderate certainty due to risk of bias, inconsistency, and imprecision [92].
Resource Implications: The 2024 Endocrine Society guideline suggests against routine 25(OH)D testing, citing cost-effectiveness considerations [10] [100] [92].
Equity and Acceptability: Empiric supplementation is generally considered feasible, acceptable, and equitable [92], though specific populations may require targeted approaches.
Implementation Considerations for Researchers
- Population Heterogeneity: Effects may differ in vitamin D-deficient versus replete individuals [45] [90]
- Dosing Regimen Importance: Daily dosing appears superior to intermittent high-dose regimens [45] [92]
- Combination Therapy: Limited evidence for vitamin D alone versus with calcium [101] [45]
- Outcome Selection: Fractures versus falls as primary endpoints may yield different conclusions [101]
The evolution of vitamin D guidelines for older adults exemplifies the complex translation of evidence into practice. While recent high-quality RCTs and meta-analyses have largely demonstrated null effects for vitamin D supplementation in community-dwelling older adults, conditional recommendations persist for specific subpopulations and indications. The evidence synthesis methods and protocols detailed herein provide researchers with robust methodologies for continuing to refine our understanding of vitamin D's role in healthy aging.
Critical research gaps remain, including optimal dosing for specific subpopulations, long-term safety of various regimens, and efficacy in underrepresented demographic groups. Future studies should prioritize personalized approaches that account for baseline vitamin D status, genetic factors, and specific clinical characteristics rather than universal supplementation strategies.
The evolution of vitamin D guidelines from the Endocrine Society's 2011 recommendations to the 2024 clinical practice guideline represents a significant paradigm shift in nutritional science and preventive medicine. The 2011 guideline primarily addressed the evaluation, treatment, and prevention of vitamin D deficiency in clinical populations [94] [102], while the 2024 guideline focuses specifically on vitamin D's role in disease prevention in "generally healthy" individuals without established indications for vitamin D treatment or testing [94] [92]. This transition reflects more than a decade of accumulated evidence from large-scale randomized controlled trials and exposes critical tensions in nutritional research methodology, particularly concerning the relative value of association studies versus interventional trials for establishing causal relationships and informing public health recommendations.
This comparative analysis examines the fundamental philosophical and methodological differences between these guideline versions, with particular emphasis on implications for research involving older adults. The 2024 guideline's more restrained approach, which narrows the populations recommended for empiric supplementation above recommended dietary intakes, underscores a maturation of evidence in the field while simultaneously highlighting significant evidence gaps that require further investigation [94] [12] [92].
Methodological Foundations: Contrasting Approaches to Evidence Evaluation
Divergent Evidence Assessment Frameworks
The 2011 and 2024 guidelines employed fundamentally different approaches to evidence evaluation, reflecting evolving standards in evidence-based medicine. The 2011 guideline utilized a comprehensive evidence base that included randomized controlled trials, association studies, and mechanistic investigations to establish vitamin D's role in bone health and beyond [102] [103]. This approach acknowledged the biological plausibility of vitamin D's extraskeletal effects while recognizing the practical challenges of conducting long-term nutrient intervention studies.
In contrast, the 2024 guideline implemented a more restrictive methodology, prioritizing data from randomized placebo-controlled trials in general populations without established indications for vitamin D treatment [92]. This approach explicitly excluded association studies and focused on "empiric supplementation" defined as vitamin D intake that exceeds Dietary Reference Intakes without prior 25-hydroxyvitamin D testing [92]. The 2024 panel leveraged the GRADE methodology to assess evidence certainty, resulting in most recommendations being classified as "conditional" due to the "low or very low certainty" of the available evidence [94].
Implications of Methodological Differences
The methodological divergence between the two guidelines has profound implications for both clinical practice and research design. The 2011 guideline's broader evidence base supported maintaining circulating 25-hydroxyvitamin D levels at least 30 ng/mL, with a preferred range of 40-60 ng/mL for maximum extraskeletal benefits [102] [103]. The 2024 guideline, constrained by the lack of supportive RCT evidence, explicitly does not endorse specific 25-hydroxyvitamin D target levels for disease prevention and suggests against routine testing in healthy populations [94] [92].
This evolution represents a classic tension in nutritional science between biological plausibility (supported by association studies and mechanistic research) and evidence of efficacy from interventional trials. As noted in critical appraisals of the 2024 guideline, this RCT-focused approach may overlook important signals from observational studies and fails to establish optimal 25-hydroxyvitamin D levels for specific health outcomes [102] [103].
Table 1: Methodological Comparison of 2011 vs. 2024 Vitamin D Guidelines
| Methodological Feature | 2011 Guideline | 2024 Guideline |
|---|---|---|
| Primary Scope | Evaluation, treatment, prevention of deficiency in patients at risk [94] [102] | Disease prevention in "generally healthy" populations [94] [92] |
| Evidence Base | RCTs, association studies, mechanistic research [102] [103] | Primarily randomized placebo-controlled trials [92] |
| 25(OH)D Level Recommendations | Specific cutoffs: deficiency (<20 ng/mL), insufficiency (21-29 ng/mL), sufficiency (30-100 ng/mL) [94] | No specific target levels endorsed for disease prevention [94] |
| Certainty of Evidence | Not formally graded using GRADE | Mostly "low" or "very low" certainty per GRADE methodology [94] |
| Recommendation Strength | Not specified | Most recommendations "conditional" [94] |
Conceptual Framework and Visual Guide to the Guideline Evolution
The following diagram illustrates the conceptual shift in approach between the 2011 and 2024 guidelines, highlighting their different scopes, evidence bases, and target populations.
Quantitative Recommendations: Comparative Analysis of Dosages and Target Populations
Population-Specific Recommendations Across Guidelines
The 2011 and 2024 guidelines demonstrate both convergence and divergence in their population-specific recommendations. The most striking differences emerge in their approaches to testing, target serum levels, and specific population recommendations, particularly for older adults.
Table 2: Comparison of Vitamin D Recommendations by Population Group
| Population Group | 2011 Guideline Recommendations | 2024 Guideline Recommendations |
|---|---|---|
| Children & Adolescents (1-18 years) | 600 IU/day; 2000 IU/d or 50,000 IU/week for treatment; maintenance 600-1000 IU/d [94] | Empiric supplementation recommended to prevent rickets and lower respiratory infection risk [94] [12] |
| Adults (19-70 years) | 600 IU/day*; 6000 IU/d or 50,000 IU/week for 8 weeks for deficiency; maintenance 1500-2000 IU/d [94] | No empiric supplementation beyond RDI for healthy adults <75 years [94] [12] |
| Adults (>70/75 years) | 800 IU/day; similar treatment dosing as younger adults [94] | Empiric supplementation recommended for >75 years for potential mortality reduction [94] [12] |
| Pregnancy | Included in adult recommendations (600 IU/day) [94] | Empiric supplementation recommended for potential reduction in preeclampsia, mortality, preterm birth [94] [12] |
| Prediabetes | Not specifically addressed | Empiric supplementation recommended for potential diabetes risk reduction [94] [12] |
| Testing Approach | Serum 25(OH)D measurement recommended for at-risk patients [104] | Against routine 25(OH)D testing in studied populations [94] [92] |
*Includes pregnant and lactating women in 2011 guideline
Dosing Strategies and Treatment Approaches
The guidelines differ substantially in their approaches to dosing strategies, particularly for deficiency treatment and maintenance therapy. The 2011 guideline provided explicit treatment protocols for deficient individuals, including high-dose loading regimens (50,000 IU weekly for 6-8 weeks) followed by maintenance therapy (1,500-2,000 IU daily) [94]. The 2024 guideline suggests daily, lower-dose vitamin D instead of non-daily, higher-dose regimens for adults aged 50 years and older with indications for supplementation [94] [92], but offers limited specific dosing guidance for most populations due to the variability in doses used in clinical trials.
This lack of specificity in the 2024 guideline, particularly regarding optimal dosing for different health outcomes, represents a significant research gap. The guideline notes that "the optimal doses for empiric vitamin D supplementation remain unclear for the populations considered" [92], reflecting the heterogeneity in dosing approaches across the clinical trials that informed the recommendations.
Implications for Older Adult Research: Special Considerations and Evidence Gaps
Age-Specific Recommendations and Rationale
The 2024 guideline introduces a critical distinction between younger and older adults that was less pronounced in the 2011 recommendations. While the 2011 guideline recommended 800 IU/day for adults aged 70+ years [94], the 2024 guideline specifically recommends empiric supplementation for adults over 75 years based on potential mortality reduction [94] [12]. This age-based stratification reflects emerging evidence that the oldest adults may derive particular benefit from vitamin D supplementation, possibly due to age-related reductions in cutaneous synthesis, decreased outdoor activity, and potential alterations in vitamin D metabolism.
Research involving older adults with obesity suggests that vitamin D supplementation at recommended levels (600 IU/day) may decrease blood pressure, with higher doses (3,750 IU/day) providing no additional benefit [105]. This finding highlights the importance of dose-response studies specifically in older populations, as aging may alter the pharmacokinetics and pharmacodynamics of vitamin D supplementation.
Movement Disorders and Neurological Considerations
Emerging research suggests potential applications for vitamin D supplementation in age-related neurological conditions, though the evidence remains preliminary. A 2024 systematic review identified four studies on Parkinson's disease and one on restless legs syndrome, noting that three of the four PD studies showed positive outcomes on measures such as decreasing levodopa-induced dyskinesia or enhancing physical performance [106]. However, the review highlighted major limitations including small sample sizes, methodological heterogeneity, and short intervention durations, preventing firm recommendations.
The biological plausibility for vitamin D's role in movement disorders stems from the presence of vitamin D receptors in brain regions including the substantia nigra, subcortical gray nuclei, and thalamus, where vitamin D influences neuronal maturation, differentiation, and neurotransmitter synthesis [106]. Additionally, vitamin D's effects on muscle strength, balance, and coordination through skeletal muscle VDRs may indirectly benefit motor symptoms in older adults with movement disorders.
Diabetes Prevention in Older Adults
The 2024 guideline's recommendation for vitamin D supplementation in high-risk prediabetes requires careful interpretation in the context of older adult research. The Finnish Vitamin D Trial (FIND), a 5-year randomized controlled trial among older adults (≥60 years for men, ≥65 years for women), found that vitamin D3 supplementation at 1600 IU/day or 3200 IU/day did not significantly reduce diabetes incidence compared to placebo in a generally healthy population with sufficient baseline vitamin D levels [107]. However, a potential beneficial effect was observed in participants with normal BMI, suggesting possible effect modification by body composition [107].
This nuanced finding highlights the importance of considering effect modifiers in older adult research and suggests that vitamin D supplementation for diabetes prevention may be most effective in specific subpopulations rather than generally healthy older adults. The 2024 guideline's specific focus on "high-risk prediabetes" rather than general population supplementation reflects this complexity [12] [92].
Experimental Protocols and Research Methodologies
Protocol for Vitamin D Intervention Studies in Older Adults
Based on analysis of the cited research, the following protocol provides a framework for conducting vitamin D intervention studies in older adult populations:
Study Population: Recruit older adults (≥65 years) with stratification by age subgroups (65-74, 75-84, ≥85), BMI categories, and baseline vitamin D status. Exclusion criteria should include conditions affecting vitamin D metabolism (renal/hepatic impairment, malabsorption), current high-dose vitamin D supplementation, and medications interfering with vitamin D metabolism [94] [107].
Intervention Design: Implement a randomized, placebo-controlled, parallel-group design with three arms: placebo, moderate-dose vitamin D3 (1600 IU/day), and higher-dose vitamin D3 (3200 IU/day) [107]. Allow continuation of personal low-dose vitamin D supplements (≤600 IU/day) to reflect real-world practices [92].
Duration and Follow-up: Maintain intervention for 12-24 months with assessments at baseline, 6, 12, and 24 months. For longer-term outcomes (fractures, diabetes incidence, mortality), extend follow-up to 3-5 years [107].
Primary Outcomes: Include both skeletal (bone mineral density, falls, fractures) and non-skeletal (mortality, infection incidence, physical function) endpoints based on population characteristics [94] [92].
Laboratory Assessments: Measure serum 25-hydroxyvitamin D3, PTH, calcium, and creatinine at all timepoints. Consider additional biomarkers relevant to specific research questions (e.g., HbA1c for diabetes prevention, inflammatory markers) [107].
Statistical Considerations: Power calculations should account for expected attrition in older populations. Include pre-specified subgroup analyses by age, BMI, baseline vitamin D status, and comorbidities [107].
Research Reagent Solutions for Vitamin D Studies
Table 3: Essential Research Reagents for Vitamin D Clinical Studies
| Reagent/Material | Specification/Function | Research Application |
|---|---|---|
| Vitamin D3 (Cholecalciferol) | Pharmaceutical grade; provided in standardized oral formulations (softgel capsules, tablets, or oil drops) [107] | Active intervention in clinical trials; ensures precise dosing and bioavailability |
| Placebo | Identical in appearance and composition to active intervention minus vitamin D3 [107] | Control condition for blinding and accounting for placebo effects |
| 25-Hydroxyvitamin D Assay | Standardized, reliable assay (e.g., LC-MS/MS preferred for reference method) [104] | Primary assessment of vitamin D status; essential for monitoring compliance and dose-response |
| Parathyroid Hormone (PTH) Assay | Immunoassay measuring intact PTH | Functional biomarker of vitamin D's calcemic effects; secondary endpoint |
| Calcium, Phosphate, Creatinine Assays | Standard clinical chemistry platforms | Safety monitoring for hypercalcemia, renal function |
| DNA Collection Kits | Standard blood or saliva collection with appropriate storage | Genetic studies of vitamin D metabolism variants (e.g., CYP2R1, GC, VDR) |
Methodological Workflow for Vitamin D Clinical Trials
The following diagram illustrates a standardized methodological approach for conducting vitamin D clinical trials in older adults, based on analysis of the studies cited in the guidelines.
The evolution from the 2011 to 2024 vitamin D guidelines reflects both advances in evidence and persistent knowledge gaps, particularly regarding older adults. Future research should prioritize several key areas:
First, dose-response relationships require clarification across different older adult subpopulations, especially for non-skeletal outcomes. The finding that higher doses (3,750 IU/day) provided no additional blood pressure benefit over 600 IU/day in older adults with obesity [105] highlights the potential for U-shaped or threshold effects that may differ by outcome and population.
Second, effect modifiers such as age, BMI, baseline vitamin D status, comorbidities, and genetic factors demand systematic investigation. The FIND trial's suggestion of potential benefit in normal-weight but not overweight/obese participants [107] illustrates the importance of moving beyond one-size-fits-all supplementation approaches.
Third, optimal trial design for nutrient studies requires innovation, including adaptive designs, enrichment strategies targeting deficient populations, and longer intervention durations to capture clinically meaningful endpoints. The limitations of existing RCTs—including sufficient baseline vitamin D levels in many participants and allowance for background supplement use—have complicated interpretation of trial results [92].
Finally, mechanistic studies embedded within clinical trials could elucidate the pathways through which vitamin D influences various health outcomes, helping to reconcile discrepancies between observational and interventional evidence. As one critical appraisal noted, "Association studies have suggested that to obtain maximum extraskeletal benefits from vitamin D... circulating concentrations of 25-hydroxyvitamin D should be at least 30 ng/mL" [102], yet RCT evidence supporting this threshold for disease prevention remains limited.
The 2024 guideline represents not an endpoint but an inflection point in vitamin D research, particularly for older adults. By addressing these research priorities, future studies can build upon this evolving evidence base to develop more targeted, effective vitamin D recommendations that account for the heterogeneity of aging populations.
Application Note: Analysis of Clinical Guideline Inconsistencies
Vitamin D supplementation and screening practices represent a significant area of clinical controversy, particularly for the growing population of older adults. Amidst increasing observational data and clinical trials with varied outcomes, numerous professional societies have developed clinical guidelines with sometimes conflicting recommendations [18]. This application note systematically analyzes the inconsistencies in vitamin D screening and supplementation guidelines for older adults, differentiating between those with and without established risk factors for vitamin D deficiency, osteoporosis, or fractures. The analysis is framed within broader research on vitamin D supplementation guidelines for older adults, providing researchers and drug development professionals with a clear comparison of current evidence-based positions and methodological approaches for evaluating clinical recommendations.
Comparative Analysis of Major Guidelines
Table 1: Comparative Vitamin D Screening Recommendations for Older Adults
| Organization/Guideline | General Older Adult Population (No Specific Risk Factors) | Older Adults with Established Risk Factors | Recommended Serum 25(OH)D Threshold |
|---|---|---|---|
| U.S. Preventive Services Task Force (USPSTF) | Recommends against routine screening [24] | Recommends against routine screening; applies to asymptomatic adults without known deficiency [24] | Not specified |
| Endocrine Society (2024) | Suggests against routine testing [108] [12] | Suggests against routine testing, even with obesity or dark complexion [12] | Outcome-specific levels not identified |
| Systematic Review of Guidelines (2025) | No identified guideline recommends screening for the general population [18] | Two-thirds of guidelines recommend screening for people at risk (e.g., osteoporosis, malabsorption, limited sun exposure) [18] | Minimum 50-75 nmol/L for at-risk groups [18] |
| International Osteoporosis Foundation (IOF) | Does not recommend universal screening [38] | Recommends measurement should be targeted at those with risk factors for severe deficiency [38] | 50 nmol/L for bone health and fracture prevention [38] |
Table 2: Comparative Vitamin D Supplementation Recommendations for Older Adults
| Organization/Guideline | General Older Adult Population (No Specific Risk Factors) | Older Adults with Osteoporosis/Fracture Risk | Recommended Daily Dose for Older Adults |
|---|---|---|---|
| U.S. Preventive Services Task Force (USPSTF) | Recommends against supplementation for primary prevention of fractures and falls (age ≥60) [24] | Recommendations do not apply to persons with osteoporosis or fracture history [24] | Not applicable (against supplementation) |
| Endocrine Society (2024) | For <75: RDA (600-800 IU). For ≥75: higher than RDA to lower mortality [108] [12] | Recommendations focus on generally healthy populations; patients with bone diseases are a distinct group [12] | ≥75 years: Above 800 IU [12] |
| National Academy of Medicine (NAM) | 600 IU/day (61-70 years); 800 IU/day (71+ years) [38] | Not specified (general population focus) | 600-800 IU (age-dependent) [38] |
| International Osteoporosis Foundation (IOF) | 800-1000 IU/day for those aged 60+ [38] | 800-1000 IU/day for all individuals with osteoporosis [38] | 800-1000 IU [38] |
| Bone Health & Osteoporosis Foundation (BHOF) | 800-1,000 IU daily for adults age 50 and older [109] | People with osteoporosis should discuss levels with provider; may need higher doses to reach 30-60 ng/mL [109] | 800-1,000 IU (general), possibly more for deficiency [109] |
Key Inconsistencies and Research Gaps
The comparative analysis reveals several critical inconsistencies across guidelines. The most significant discrepancy exists between guidelines focused on general disease prevention versus those focused on bone health specialty care. The USPSTF gives a "recommendation against" vitamin D supplementation for primary prevention of fractures and falls in community-dwelling adults aged 60 and older, concluding with moderate certainty that it has no net benefit [24]. Conversely, the IOF and BHOF, which specialize in bone health, recommend daily supplementation of 800-1000 IU for adults over 60 or with osteoporosis to support bone health and reduce fall risk [38] [109].
A second major inconsistency involves the definition of the target "older adult" population. The Endocrine Society delineates its recommendations by specific age thresholds (under 75 vs. 75 and older) [12], while the USPSTF uses age 60 and the IOF uses age 60 as the threshold for higher recommendations [24] [38]. Furthermore, the Endocrine Society's 2024 guideline identifies specific populations that may benefit from higher-than-RDA supplementation, including adults over 75 for mortality risk reduction and people with prediabetes to reduce diabetes progression [108] [12].
The evidence base for recommendations also varies substantially. The USPSTF and the systematic review note that many large clinical trials were not designed for all the outcomes they reported, and studied populations often had adequate vitamin D levels at baseline [24] [18]. This has led to significant limitations in establishing specific blood-level thresholds for disease prevention.
Diagram 1: Guideline Decision Pathway for Older Adults
Experimental Protocols
Protocol 1: Systematic Guideline Review and Analysis
Objective: To systematically identify, appraise, and compare clinical practice guidelines for vitamin D screening and supplementation in older adult populations with and without established risk factors.
Materials:
- Database Sources: PubMed, Embase (Ovid), Cochrane Reviews, Google Scholar, and guideline repositories from the USPSTF, Endocrine Society, and NICE.
- Search Filter: Canadian Agency for Drugs and Technologies in Health (CADTH) standardized clinical practice guideline filters for high sensitivity retrieval.
- Quality Appraisal Tool: AGREE II (Appraisal of Guidelines Research and Evaluation version 2) instrument.
- Data Extraction: Standardized electronic form for recording recommendations, target populations, dosage, evidence quality, and conflicts of interest.
Methodology:
- Search Strategy: Execute a systematic search for guidelines published from January 2013 to June 2024 using the validated CADTH guideline filters with terms "vitamin D," "guideline," "screening," "supplementation," and "older adults."
- Screening Process: Two independent reviewers screen titles/abstracts using Rayyan software, followed by full-text review against predefined inclusion/exclusion criteria.
- Data Extraction: Extract data on publishing organization, publication year, target population, screening recommendations, supplementation dosage, serum level targets, and strength of evidence using a standardized electronic form.
- Quality Appraisal: Assess methodological rigor of included guidelines using AGREE II across six domains by two independent reviewers.
- Comparative Analysis: Synthesize data in comparative tables and identify areas of consistency and inconsistency, particularly regarding risk-based versus age-based recommendations.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Guideline Review and Clinical Research
| Item | Function/Application in Research |
|---|---|
| CADTH Guideline Filters | Validated search filters to identify clinical practice guidelines with high sensitivity in biomedical databases. |
| AGREE II Instrument | Standardized tool to appraise the methodological quality and transparency of guideline development processes. |
| Rayyan Software | Web-based systematic review tool for collaborative screening and selection of studies during review process. |
| Serum 25-Hydroxyvitamin D Assays | Gold-standard measurement for determining vitamin D status in clinical trials and observational studies. |
| Vitamin D3 (Cholecalciferol) Supplements | The most commonly used and often recommended form of vitamin D for supplementation studies in older adults. |
Protocol 2: In Silico Analysis of Recommendation Patterns
Objective: To quantitatively analyze the relationship between guideline recommendations, supporting evidence hierarchy, and organizational specialty focus using computational methods.
Materials:
- Guideline Corpus: Digitized full-text versions of all included guidelines from Protocol 1.
- Text Mining Software: Natural language processing tools for recommendation extraction and classification.
- Statistical Analysis Platform: R or Python with appropriate statistical packages for correlation and regression analysis.
- Evidence Classification Schema: Standardized system for categorizing evidence quality (e.g., RCTs, meta-analyses, observational data).
Methodology:
- Recommendation Coding: Develop a coding framework to classify each recommendation by direction (for/against), strength (strong/weak), target population, and dosage.
- Evidence Mapping: Extract and categorize the cited evidence for each recommendation according to study design, sample size, population characteristics, and outcomes measured.
- Correlation Analysis: Examine statistical relationships between guideline characteristics (organizational specialty, development methodology) and recommendation outcomes using appropriate statistical tests.
- Network Analysis: Construct and visualize networks connecting guidelines, evidence sources, and recommendation patterns to identify influential studies and consensus clusters.
- Gap Analysis: Identify areas where multiple guidelines cite insufficient evidence, highlighting priorities for future clinical research.
Diagram 2: In Silico Analysis Workflow
Discussion & Research Implications
Interpretation of Findings
The identified inconsistencies stem from several fundamental sources. First, guidelines interpret clinical trial evidence differently, particularly regarding the inclusion of participants with sufficient baseline vitamin D levels, which may obscure potential benefits for truly deficient populations [110]. Second, the scope and target population varies significantly, with general preventive guidelines (USPSTF) focusing on otherwise healthy community-dwelling adults, while specialty guidelines (IOF, BHOF) target patients with or at high risk for bone disease [24] [38] [109].
Third, methodological challenges in vitamin D research contribute to divergent interpretations. As noted in commentary on the Endocrine Society guideline, control groups in vitamin D trials typically receive some vitamin D from sunshine and diet, potentially diminishing measured treatment effects [110]. Additionally, many large trials were not specifically designed for all reported outcomes [12].
This analysis demonstrates clear inconsistencies in vitamin D screening and supplementation recommendations for older adults, primarily divided between general preventive medicine and bone health specialty perspectives. These discrepancies present significant challenges for clinicians, policymakers, and drug development professionals seeking evidence-based guidance.
Future research should prioritize:
- Risk-stratified clinical trials specifically enrolling older adults with confirmed vitamin D deficiency at baseline.
- Harmonization of outcome measures across trials to enable more meaningful meta-analyses.
- Individual participant data meta-analyses to identify potential subgroups that may benefit from supplementation.
- Cost-effectiveness analyses of different screening strategies targeted to high-risk older adult populations.
Addressing these research gaps will facilitate the development of more unified, evidence-based guidelines that can optimize vitamin D-related care for the heterogeneous population of older adults.
Conclusion
Current clinical practice guidelines for vitamin D supplementation in older adults converge on recommended daily intakes of 800 IU or more but reveal significant disparities in optimal dosing, target serum levels, and screening protocols. The 2024 Endocrine Society guideline reframes supplementation around disease prevention in generally healthy individuals, yet critical evidence gaps persist, particularly for non-skeletal outcomes and personalized dosing strategies. Future biomedical research must prioritize large-scale, long-term randomized controlled trials to establish causal benefits for mortality, cognitive function, and immune health. For drug development, opportunities exist in formulating improved delivery systems to enhance adherence and exploring biomarkers beyond 25(OH)D for better treatment monitoring. The decline in recent vitamin D research funding and publication momentum underscores an urgent need for reinvestment to resolve these unanswered questions and optimize therapeutic approaches for the rapidly aging global population.
References
- 1. Vitamin D and the aging skin: insights into oxidative stress ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12584448/]
- 2. Older adults at risk of vitamin D deficiency [https://www.uclahealth.org/news/article/older-adults-risk-vitamin-d-deficiency]
- 3. Vitamin D promotes wound healing in aged skin by ... [https://www.sciencedirect.com/science/article/abs/pii/S096007602500127X]
- 4. Vitamin D and your health: Breaking old rules, raising new ... [https://www.health.harvard.edu/staying-healthy/vitamin-d-and-your-health-breaking-old-rules-raising-new-hopes]
- 5. The Role of Vitamin D in Health and Aging [https://www.uspharmacist.com/article/the-role-of-vitamin-d-in-health-and-aging]
- 6. This everyday vitamin could be the closest thing we have to ... [https://www.sciencedaily.com/releases/2025/10/251022023132.htm]
- 7. Vitamin D Supplements Show Signs of Protection Against ... [https://www.massgeneralbrigham.org/en/about/newsroom/press-releases/vitamin-d-supplements-show-signs-of-protection-against-biological-aging]
- 8. Individual and additive effects of vitamin D, omega-3 and ... [https://www.nature.com/articles/s43587-024-00793-y]
- 9. The Optimal Protective 25-Hydroxyvitamin D Level for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12029153/]
- 10. Vitamin D for the Prevention of Disease Guideline Resources [https://www.endocrine.org/clinical-practice-guidelines/vitamin-d-for-prevention-of-disease]
- 11. Vitamin D deficiency 2.0: an update on the current status ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7091696/]
- 12. Endocrine Society Guideline recommends healthy adults ... [https://www.endocrine.org/news-and-advocacy/news-room/2024/endocrine-society-recommends-healthy-adults-take-the-recommended-daily-allowance-of-vitamin-d]
- 13. New clinical practice guidelines for vitamin D ... [https://myadlm.org/science-and-research/clinical-chemistry/clinical-chemistry-podcasts/2025/new-clinical-practice-guidelines-for-vitamin-d-supplementation-and-testing]
- 14. Vitamin D sufficiency and its relationship with muscle ... [https://www.nature.com/articles/s41430-025-01610-4]
- 15. Cutoff levels of serum 25-OH-D for defining vitamin D ... [https://www.sciencedirect.com/science/article/pii/S1875957225000191]
- 16. Different diagnostic criteria influence the determination of ... [https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2025.1641065/full]
- 17. High Prevalence and Clinical Associations of Vitamin D ... [https://www.mdpi.com/2072-6643/17/23/3698]
- 18. A systematic review of evidence-based clinical guidelines for ... [https://archpublichealth.biomedcentral.com/articles/10.1186/s13690-025-01709-x]
- 19. Muscle function mediates the association between vitamin ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12626798/]
- 20. Vitamin D and Sarcopenia in the Senior People [https://pmc.ncbi.nlm.nih.gov/articles/PMC11382659/]
- 21. Individual and joint association of serum 25-hydroxyvitamin ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2025.1576705/full]
- 22. Anti-sarcopenic effects of active vitamin D through ... [https://www.sciencedirect.com/science/article/pii/S002604952500109X]
- 23. Vitamin D and Calcium in Osteoporosis, and the Role of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9944083/]
- 24. Draft Recommendation: Vitamin D, Calcium, or Combined ... [https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/vitamin-d-calcium-combined-supplementation-primary-prevention-falls-fractures-communitydwelling-adults]
- 25. Vitamin D and Sarcopenia: Implications for Muscle Health - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12383353/]
- 26. Impact of Vitamin D and Calcium on Falls and Fractures in ... [https://www.sciencedirect.com/science/article/pii/S1530891X25009656]
- 27. Vitamin D in Autoimmunity: Molecular Mechanisms and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5247472/]
- 28. The role of vitamin D in autoimmune diseases [https://bsd.biomedcentral.com/articles/10.1186/s13293-021-00358-3]
- 29. High-dose Vitamin D supplementation for immune ... [https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1625769/full]
- 30. Higher serum vitamin D concentrations are associated with ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2196219/]
- 31. Associations between vitamin D and autoimmune diseases [https://www.sciencedirect.com/science/article/pii/S004901722300080X]
- 32. Vitamin D Supplements Shows Promise for Slowing Aging ... [https://www.uspharmacist.com/article/vitamin-d-supplements-shows-promise-for-slowing-aging-process]
- 33. Will vitamin D supplements keep me younger? [https://www.health.harvard.edu/staying-healthy/will-vitamin-d-supplements-keep-me-younger]
- 34. Global and regional prevalence of vitamin D deficiency in ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2023.1070808/full]
- 35. Global prevalence and disease burden of vitamin D deficiency [https://pmc.ncbi.nlm.nih.gov/articles/PMC7309365/]
- 36. The Prevalence and Determinants of Vitamin D Status ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10080568/]
- 37. Global and regional prevalence of vitamin D deficiency in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10064807/]
- 38. Vitamin D recommendations [https://www.osteoporosis.foundation/vitamin-d-recommendations]
- 39. Prevalence and patterns of vitamin D deficiency and its role ... [https://www.nature.com/articles/s41598-024-62010-5]
- 40. Vitamin D Deficiency and Clinical Outcomes in Critically Ill ... [https://pubmed.ncbi.nlm.nih.gov/40242208/]
- 41. A systematic review of evidence-based clinical guidelines ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12395781/]
- 42. Vitamin D Information | Mount Sinai - New York [https://www.mountsinai.org/health-library/nutrition/vitamin-d]
- 43. Vitamin D - The Nutrition Source [https://nutritionsource.hsph.harvard.edu/vitamin-d/]
- 44. Get the Facts on Calcium and Vitamin D [https://www.bonehealthandosteoporosis.org/patients/treatment/calciumvitamin-d/get-the-facts-on-calcium-and-vitamin-d/]
- 45. Effect of vitamin D, calcium, or combined supplementation on ... [https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-024-05009-x]
- 46. Vitamin D Supplementation Is Associated with a Reduction ... [https://www.sciencedirect.com/science/article/pii/S2260134124001452]
- 47. Impact of vitamin D supplementation on cognitive ... [https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2025.1571078/full]
- 48. Effects of vitamin D supplementation in patients with ... [https://www.sciencedirect.com/science/article/pii/S2405844025008436]
- 49. Impact of vitamin D supplementation on disease activity ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12247244/]
- 50. The Effect of Vitamin D Supplementation on Clinical and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12536741/]
- 51. The Effect of Vitamin D Supplementation on Rheumatoid ... [https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2020.596007/full]
- 52. Vitamin D [https://www.mayoclinic.org/drugs-supplements-vitamin-d/art-20363792]
- 53. New Vitamin D Guidelines: Who Really Needs Supplements? [https://healthcare.utah.edu/the-scope/health-library/all/2024/12/new-vitamin-d-guidelines-who-really-needs-supplements]
- 54. Vitamin D in the older population: a consensus statement - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9607753/]
- 55. Randomised Controlled Trial Comparing Daily Versus ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5872766/]
- 56. Efficacy of different doses and time intervals of oral vitamin D ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2277446/]
- 57. Efficacy of intermittent versus daily vitamin D supplementation ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10488712/]
- 58. Supplementing Vitamin D in Different Patient Groups to ... [https://www.mdpi.com/2072-6643/15/17/3725]
- 59. Randomised Controlled Trial Comparing Daily Versus ... [https://www.mdpi.com/2072-6643/10/3/348]
- 60. Efficacy of daily 800 IU vitamin D supplementation in reaching ... [https://bmcgeriatr.biomedcentral.com/articles/10.1186/1471-2318-14-103]
- 61. Vitamin D: Evidence-Based Health Benefits and ... [https://www.mdpi.com/2072-6643/17/2/277]
- 62. Efficacy of Vitamin D Supplementation on the Risk of Falls ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12429313/]
- 63. Efficacy of Vitamin D Supplementation on the Risk of Falls ... [https://www.mdpi.com/2077-0383/14/17/6117]
- 64. Safety and Efficacy of Loading Doses of Vitamin D [https://www.mdpi.com/1424-8247/17/12/1620]
- 65. Vitamin D supplementation: Pearls for practicing clinicians [https://www.ccjm.org/content/89/3/154]
- 66. High single doses of vitamin D via food supplements at ... [https://www.bfr.bund.de/en/opinions/high-single-doses-of-vitamin-d-via-food-supplements-at-intervals-of-days-or-weeks-associated-with-health-risks/]
- 67. Vitamin D3 25-Hydroxyvitamin D [https://emedicine.medscape.com/article/2088694-overview]
- 68. Redefining vitamin D status: Establishing population-based ... [https://www.sciencedirect.com/science/article/abs/pii/S0009898125000348]
- 69. Optimizing Vitamin D Levels: A Real-World Analysis of ... [https://schw-aerztej.healthbooktimes.org/article/133945-optimizing-vitamin-d-levels-a-real-world-analysis-of-supplementation-efficacy-and-patient-outcomes]
- 70. Monitoring changes in vitamin D levels during the COVID ... [https://www.nature.com/articles/s41467-025-64192-6]
- 71. A systematic review of evidence-based clinical guidelines ... [https://pubmed.ncbi.nlm.nih.gov/40883815/]
- 72. How Follow-Up Period in Prospective Cohort Studies Affects ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11547645/]
- 73. Inappropriate vitamin D supplementation among ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12275266/]
- 74. Associations of endocrine disrupting chemical biomarkers ... [https://www.sciencedirect.com/science/article/pii/S0013935125025691]
- 75. Association of vitamin A and D deficiency and the presence ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2025.1676174/full]
- 76. Prevalence and determinants of profound vitamin D ... [https://www.sciencedirect.com/science/article/pii/S0960076025000652]
- 77. Vitamin D Deficiency - StatPearls - NCBI Bookshelf [https://www.ncbi.nlm.nih.gov/books/NBK532266/]
- 78. Daily and Weekly “High Doses” of Cholecalciferol for the ... [https://www.mdpi.com/2072-6643/16/15/2541]
- 79. Daily and Weekly “High Doses” of Cholecalciferol for the ... [https://www.preprints.org/manuscript/202407.1527/v1]
- 80. Understanding the Endocrine Impact of Vitamin D, Calcium ... [https://consultqd.clevelandclinic.org/understanding-the-endocrine-impact-of-vitamin-d-calcium-deficiency-in-the-elderly]
- 81. Unintended Consequences of Obesity Pharmacotherapy [https://pmc.ncbi.nlm.nih.gov/articles/PMC12157928/]
- 82. Medication non-adherence: reflecting on two decades since ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11700791/]
- 83. Barriers and Facilitators to Medication Adherence among ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11395048/]
- 84. Vitamin D Supplementation for Infants [https://www.who.int/tools/elena/bbc/vitamind-infants]
- 85. The silent epidemic of non-adherence – insights from the ... [https://bmcproc.biomedcentral.com/articles/10.1186/s12919-025-00326-4]
- 86. Oral nutritional supplement (ONS) compliance [https://www.dsm-firmenich.com/en/businesses/health-nutrition-care/news/talking-nutrition/barriers-and-drivers-for-ons-adherence/full-article.html]
- 87. An Educational Intervention to Improve Clinician Vitamin D ... [https://pubmed.ncbi.nlm.nih.gov/39753202/]
- 88. Classifying Supplement Use Status in Clinical Notes - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC5543386/]
- 89. Vitamin D supplementation: upper limit for safety revisited? [https://pmc.ncbi.nlm.nih.gov/articles/PMC7897606/]
- 90. The health effects of vitamin D supplementation: evidence ... [https://www.nature.com/articles/s41574-021-00593-z]
- 91. Comparing the Evidence from Observational Studies and ... [https://www.mdpi.com/2072-6643/14/18/3811]
- 92. Vitamin D for the Prevention of Disease: An Endocrine ... [https://pubmed.ncbi.nlm.nih.gov/38828931/]
- 93. Vitamin D supplementation: an update [https://bpac.org.nz/2025/vitamind.aspx]
- 94. Comparing 2011 & 2024 Vitamin D Guidelines [https://www.pharmacy.umn.edu/vitamin-d-dilemma-comparing-2011-2024-vitamin-d-guidelines]
- 95. 2025 Vitamin D Deficiency Statistics of UK Adults - Forth [https://www.forthwithlife.co.uk/blog/uk-vitamin-d-statistics/]
- 96. Vitamin D management update: evidence-based guidelines ... [https://erar.springeropen.com/articles/10.1186/s43166-025-00330-8]
- 97. Vitamin D: What's the "right" level? [https://www.health.harvard.edu/blog/vitamin-d-whats-right-level-2016121910893]
- 98. Efficacy of different doses and time intervals of oral vitamin ... [https://pubmed.ncbi.nlm.nih.gov/17874029/]
- 99. Vitamin D Certified Assays and Participants [https://www.cdc.gov/clinical-standardization-programs/php/vitamin-d/list-of-vitamin-d-certified-assays.html]
- 100. New guidelines released for vitamin D testing and supplementation - Harvard Health [https://www.health.harvard.edu/staying-healthy/new-guidelines-released-for-vitamin-d-testing-and-supplementation]
- 101. Vitamin D, Calcium, or Combined Supplementation for ... - NCBI [https://www.ncbi.nlm.nih.gov/books/NBK525395/]
- 102. Revisiting Vitamin D Guidelines: A Critical Appraisal of the ... [https://pubmed.ncbi.nlm.nih.gov/39486479/]
- 103. Revisiting Vitamin D Guidelines: A Critical Appraisal of the ... [https://www.sciencedirect.com/science/article/abs/pii/S1530891X24008048]
- 104. (Vitamin D Guidelines) Evaluation, Treatment, and ... [https://www.natap.org/2011/HIV/060911_02.htm]
- 105. Vitamin D supplements may lower blood pressure in older ... [https://www.endocrine.org/news-and-advocacy/news-room/2024/vitamin-d-supplements-may-lower-blood-pressure-in-older-people-with-obesity]
- 106. Vitamin D supplementation in later life: a systematic review ... [https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2024.1333217/full]
- 107. The effect of vitamin D 3 supplementation on the incidence ... [https://pubmed.ncbi.nlm.nih.gov/39621103/]
- 108. Endocrine Society Guideline Recommends Healthy Adults ... [https://www.icaa.cc/industrynews/2024-06/Endocrine-Society-Guideline-recommends-healthy-adults-under-the-age-of-75-take-the-recommended-daily-allowance-of-vitamin-D.htm]
- 109. Calcium/Vitamin D Requirements, Recommended Foods & ... [https://www.bonehealthandosteoporosis.org/patients/treatment/calciumvitamin-d/]
- 110. Vitamin D supplements for prevention of diseases [https://www.sciencedirect.com/science/article/pii/S0953620525002663?dgcid=rss_sd_all]