Endocrine-Disrupting Chemicals (EDCs): Exposure Routes, Health Impacts, and Risk Assessment Strategies for Biomedical Research
This article provides a comprehensive analysis of Endocrine-Disrupting Chemical (EDC) exposure routes—food, air, and skin absorption—and their implications for human health and drug development.
Endocrine-Disrupting Chemicals (EDCs): Exposure Routes, Health Impacts, and Risk Assessment Strategies for Biomedical Research
Abstract
This article provides a comprehensive analysis of Endocrine-Disrupting Chemical (EDC) exposure routes—food, air, and skin absorption—and their implications for human health and drug development. It synthesizes foundational knowledge on EDC mechanisms and sources, explores advanced methodologies for exposure assessment and biomonitoring, addresses challenges in risk evaluation and mitigation, and examines validation frameworks and regulatory landscapes. Aimed at researchers, scientists, and drug development professionals, this review consolidates current evidence to inform robust study design, risk assessment, and public health policy.
Understanding EDCs: Sources, Exposure Routes, and Mechanistic Pathways to Disease
Endocrine-disrupting chemicals (EDCs) are defined as exogenous (non-natural) chemicals, or mixtures of chemicals, that can interfere with any aspect of hormone action [1]. The endocrine system, which includes glands distributed throughout the body that produce hormones, controls critical biological processes such as normal growth, fertility, and reproduction [2]. These hormones act as signaling molecules in extremely small amounts, and even minor disruptions in their levels may cause significant developmental and biological effects [2]. EDCs include a wide array of natural and human-made substances found in pesticides, cosmetics, household cleaners, plastic containers, food packaging, fabric, upholstery, electronics, and medical equipment [3].
The global scientific community has recognized EDCs as emerging contaminants and a significant public health challenge [3]. These chemicals contaminate nearly every ecosystem tested, even in the most remote areas of the world, and are significantly associated with various neurological, neurodevelopmental, and reproductive disorders [3]. Research activity on EDCs has increased disproportionately, with a strong north-south divide in publication performance, dominated by the United States and China [3]. This technical guide provides an in-depth examination of the mechanisms through which EDCs interfere with the endocrine system, the primary routes of human exposure, and the resulting health effects, with particular focus on food, air, and skin absorption pathways.
Mechanisms of Endocrine Disruption
Key Characteristics of Endocrine-Disrupting Chemicals
Informed by the consensus of international experts, EDCs can be systematically identified and evaluated based on ten key characteristics (KCs) that reflect their ability to interfere with hormone systems [4]. These KCs provide a framework for organizing mechanistic evidence and reflect current scientific knowledge of hormone action [4].
Table 1: Ten Key Characteristics of Endocrine-Disrupting Chemicals
| Characteristic | Mechanism of Action | Representative Examples |
|---|---|---|
| KC1: Interacts with or activates hormone receptors | EDCs inappropriately bind to and/or activate hormone receptors, mimicking natural hormones | DDT binds to estrogen receptors (ERα/ERβ); hydroxylated PCBs activate thyroid hormone receptor-β [4] |
| KC2: Antagonizes hormone receptors | EDCs inhibit or block effects of endogenous hormones by acting as receptor antagonists | Organochlorine pesticides inhibit androgen binding to the androgen receptor [4] |
| KC3: Alters hormone receptor expression | EDCs modulate hormone receptor expression, internalization, and degradation | BPA alters expression of estrogen, oxytocin, and vasopressin receptors in brain nuclei [4] |
| KC4: Alters signal transduction in hormone-responsive cells | EDCs disrupt intracellular responses triggered by hormone-receptor binding | BPA blocks glucose-induced calcium signaling in pancreatic α-cells [4] |
| KC5: Induces epigenetic modifications in hormone-producing or responsive cells | EDCs cause heritable changes in gene expression without altering DNA sequence | Diethylstilbestrol (DES) causes epigenetic changes in reproductive organs of mice [2] [4] |
| KC6: Alters hormone synthesis | EDCs affect production and secretion of hormones | Perchlorate inhibits thyroidal iodide uptake and thyroid hormone synthesis [4] |
| KC7: Alters hormone transport across cell membranes | EDCs disrupt circulating hormone transport proteins | PBDEs compete with thyroid hormone for binding to transthyretin [4] |
| KC8: Alters hormone distribution or circulating levels | EDCs modify hormone metabolism and clearance | PCBs increase metabolism and clearance of thyroid hormones [4] |
| KC9: Alters fate of hormone-producing or responsive cells | EDCs affect proliferation, differentiation, or death of hormone-sensitive cells | Phthalates alter germ cell numbers and reproductive tract development [4] |
| KC10: Alters hormone-regulated physiological systems | EDCs interfere with homeostasis, reproduction, development, and/or behavior | EDC mixtures alter brain pathways controlling reward preference and eating behavior [5] |
Molecular Pathways of Endocrine Disruption
The following diagram illustrates the primary molecular pathways through which EDCs interfere with normal endocrine signaling, from cellular reception to systemic physiological effects:
EDCs employ multiple mechanisms to disrupt endocrine function, often simultaneously. They can mimic natural hormones, block hormone receptors, or alter hormone production, transport, and metabolism [6]. The mechanisms are particularly consequential during sensitive developmental windows, such as embryonic development, pregnancy, and lactation, when small disturbances in endocrine function can lead to profound and lasting effects [6].
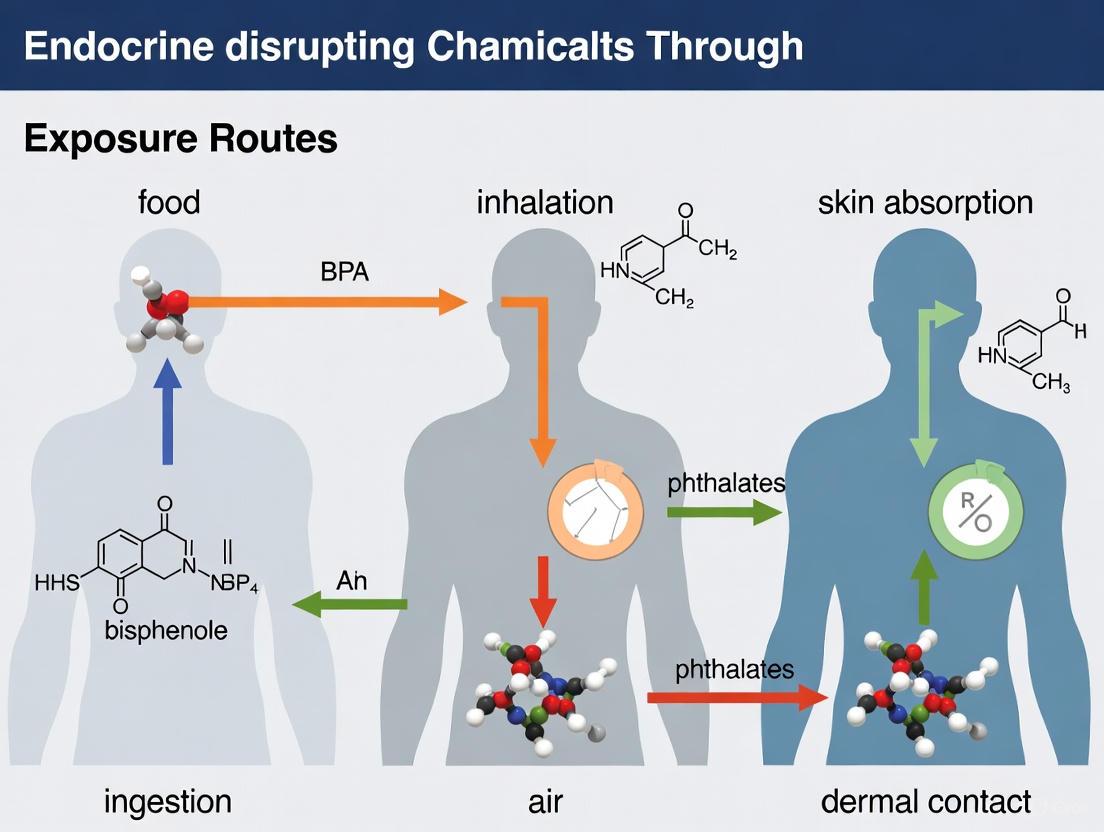
Exposure Routes and Environmental Vectors
Primary Exposure Pathways
Human exposure to EDCs occurs through three primary routes: diet, inhalation, and dermal absorption [1]. The following diagram illustrates the major exposure pathways and their connections to common EDC sources:
Dietary ingestion represents a major exposure route for numerous EDCs. Chemicals such as bisphenol A (BPA) leach from food and beverage packaging, while pesticides like atrazine and other agricultural chemicals contaminate food products [2]. Phthalates, used as liquid plasticizers, can migrate from food packaging into contents [2]. Perchlorate, an industrial chemical used in rockets, explosives, and fireworks, has been detected in some groundwater sources and can enter the food chain [2]. Dioxins, generated as byproducts of manufacturing processes and waste burning, accumulate in the food chain, particularly in animal fats [2].
Dermal absorption occurs through direct contact with personal care products, cosmetics, and household items containing EDCs. Phthalates are found in hundreds of products including cosmetics, fragrances, nail polish, hair spray, and shampoos [2]. The skin, being the main route of exposure for cosmetic products, can absorb these chemicals, especially when the skin's protective barrier is compromised [7]. An individual typically uses at least two personal care products in a 24-hour period, with between 30-40% of dermatologist prescriptions containing at least one personal care product component [1].
Inhalation exposes individuals to EDCs through airborne contaminants. Chemicals from personal care products, such as siloxanes and phthalates, can become airborne and contaminate indoor air [1]. For instance, shower gel and shampoo alone can emit significant quantities of siloxanes per individual per day [1]. Dioxins released into the air from waste burning and wildfires represent another inhalation exposure source [2]. Household dust also serves as a reservoir for EDCs like flame retardants (PBDEs) that can become airborne and inhaled [2].
Table 2: Major Endocrine-Disrupting Chemicals and Exposure Sources
| EDC Category | Common Sources | Primary Exposure Routes |
|---|---|---|
| Bisphenol A (BPA) | Polycarbonate plastics, epoxy resins, food can linings, toys, thermal paper receipts | Dietary ingestion (primary), dermal absorption [2] [1] |
| Phthalates | Food packaging, cosmetics, fragrances, children's toys, medical device tubing, personal care products | Dermal absorption, dietary ingestion, inhalation [2] [1] |
| Per- and polyfluoroalkyl substances (PFAS) | Firefighting foam, nonstick pans, paper and textile coatings, food packaging | Dietary ingestion, inhalation of household dust [2] |
| Pesticides | Agricultural applications (atrazine, DDT, glyphosate), home and garden products, contaminated food and water | Dietary ingestion, inhalation, dermal absorption [2] [8] |
| Polychlorinated biphenyls (PCBs) | Electrical equipment, hydraulic fluids, plasticizers (despite 1979 ban, persistent in environment) | Dietary ingestion (especially fish), inhalation [2] |
| Dioxins | Industrial processes, waste incineration, wildfires, herbicide production | Dietary ingestion (animal fats), inhalation [2] |
| Phytoestrogens | Naturally occurring in plants (soy foods, flaxseeds) | Dietary ingestion [2] |
| Polybrominated diphenyl ethers (PBDE) | Flame retardants in furniture foam, carpet, electronics | Inhalation of household dust, dietary ingestion [2] |
| Heavy Metals | Industrial processes, contaminated water and soil, certain foods | Dietary ingestion, inhalation [8] |
| Triclosan | Previously in antimicrobial soaps and personal care products (restricted in some regions) | Dermal absorption, inhalation [2] |
Health Effects and Associated Outcomes
Comprehensive Health Impact Analysis
An umbrella review of 67 meta-analyses encompassing 109 health outcomes from 7,552 unique articles revealed significant associations between EDC exposure and numerous adverse health conditions [8]. The analysis included pesticides (30 studies), BPA (13 studies), polycyclic aromatic hydrocarbons or PAHs (18 studies), PFAS (10 studies), and heavy metals (38 studies) [8].
Table 3: Health Outcomes Associated with EDC Exposure
| Health Category | Specific Outcomes with Significant Associations | Strength of Evidence |
|---|---|---|
| Cancer | 22 cancer outcomes including breast, prostate, testicular, and reproductive cancers | Strong evidence from multiple meta-analyses [8] |
| Reproductive Health | Infertility, endometriosis, decreased sperm quality, PCOS, premature thelarche, reproductive tract malformations | Strong consistent evidence across studies [2] [1] [6] |
| Metabolic Disorders | Obesity, diabetes, insulin resistance, metabolic syndrome | Significant associations with 18 metabolic disorder outcomes [8] |
| Neurodevelopment | ADHD, cognitive deficits, neurodevelopmental delays, autism spectrum behaviors | Strong evidence, particularly for prenatal exposures [2] [3] |
| Cardiovascular Health | Hypertension, cardiovascular disease, atherosclerosis | 17 significant cardiovascular disease outcomes [8] |
| Pregnancy & Fetal Development | Preterm birth, fetal growth restriction, implantation failure, gestational diabetes | 11 significant pregnancy-related outcomes [8] |
| Immune Function | Diminished immune response to vaccines, immune system dysfunction | Children exposed to high PFAS levels showed reduced vaccine response [2] |
| Other Systems | Renal, respiratory, and hematologic outcomes | 20 additional significant outcomes across various systems [8] |
Developmental and Transgenerational Effects
Perhaps the most concerning aspect of EDC exposure involves their impact during critical developmental windows. The developing fetus is particularly vulnerable to EDCs, which can cross the placental barrier and interfere with organ formation and differentiation [3] [6]. Exposure during these sensitive periods can lead to epigenetic changes that alter gene expression patterns and increase disease susceptibility later in life [2] [4].
The case of diethylstilbestrol (DES) exemplifies the devastating consequences of developmental EDC exposure. From the 1940s through 1970s, DES was prescribed to pregnant women to prevent miscarriage, but was later linked to vaginal cancer in daughters of women who took the drug, along with numerous noncancerous changes in both sons and daughters [2]. This experience demonstrated that EDC exposure during development can cause long-lasting health effects that manifest decades later, sometimes even in subsequent generations [2].
Recent research continues to reveal transgenerational effects of EDCs. Animal studies have shown that exposure to EDCs during gestation can lead to epigenetic transgenerational actions affecting mate fertility in subsequent generations [3]. Additionally, early-life exposure to EDCs has been linked to altered food preferences and reward pathways in the brain, potentially contributing to obesity rates [5]. University of Texas researchers found that rats exposed to EDC mixtures during gestation or infancy showed heightened preference for sugary and fatty foods later in life, with physical changes in brain regions controlling food intake and reward response [5].
Experimental Methodologies and Research Approaches
Research Workflow for EDC Hazard Assessment
The following diagram illustrates a comprehensive experimental workflow for identifying and characterizing endocrine-disrupting chemicals:
Detailed Experimental Protocols
In Vitro Receptor Binding Assays Protocol Objective: To determine the ability of test chemicals to bind to and activate or inhibit hormone receptors including estrogen receptors (ERα and ERβ), androgen receptor (AR), and thyroid hormone receptors (TRα and TRβ).
Methodology:
- Receptor Preparation: Isolate and purify recombinant human hormone receptors or use receptor-containing cell lines (e.g., MCF-7 cells for ER, HEK293 cells transfected with AR).
- Competitive Binding Assay: Incubate receptors with radiolabeled natural hormones (³H-estradiol for ER, ³H-testosterone for AR) in the presence of increasing concentrations of test chemicals.
- Separation and Measurement: Separate bound from free ligand using charcoal adsorption, filtration, or immunoprecipitation methods.
- Data Analysis: Calculate IC₅₀ values and relative binding affinity (RBA) compared to natural hormones. RBA = (IC₅₀ of reference compound/IC₅₀ of test compound) × 100.
Quality Control: Include reference EDCs (DES for ER, vinclozolin for AR) as positive controls and vehicle-only as negative control. Perform experiments in triplicate with at least three independent replicates [4].
Transcriptional Activation Assays Protocol Objective: To assess the ability of test chemicals to activate hormone-responsive gene expression.
Methodology:
- Reporter Gene Construction: Transfect cells with plasmid containing hormone response elements (ERE for estrogen, ARE for androgen) upstream of a luciferase or GFP reporter gene.
- Chemical Exposure: Expose transfected cells to a range of test chemical concentrations (typically 10⁻¹² to 10⁻⁶ M) for 24-48 hours.
- Response Measurement: Lyse cells and measure reporter gene activity using luminometry (luciferase) or fluorometry (GFP).
- Dose-Response Analysis: Calculate EC₅₀ values and efficacy relative to natural hormones.
Variations: Yeast-based reporter systems provide alternative screening platform with different permeability and metabolic properties [4].
In Vivo Pubertal Assay (EPA OPPTS 890.1450) Protocol Objective: To detect endocrine-disrupting effects on pubertal development and thyroid function.
Methodology:
- Animal Model: Weanling rats (Sprague-Dawley, Wistar, or Long-Evans) at postnatal day 21.
- Exposure Regimen: Administer test chemical orally (by gavage) or in diet for 21-31 days.
- Endpoints Measured:
- Vaginal opening (females) and preputial separation (males) as puberty markers
- Estrous cyclicity (females)
- Thyroid histopathology
- Serum T₃, T₄, and TSH levels
- Organ weights (liver, kidney, pituitary, thyroid, reproductive organs)
- Statistical Analysis: Compare treatment groups to controls using ANOVA followed by appropriate post-hoc tests.
Interpretation: Delayed or accelerated puberty suggests sex steroid-mediated effects; thyroid histopathology and hormone changes indicate thyroid disruption [6].
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Research Reagents for EDC Investigation
| Reagent/Cell Line | Application | Specific Utility in EDC Research |
|---|---|---|
| MCF-7 cells | Estrogen receptor binding and proliferation assays | Human breast cancer cell line expressing ERα; used to assess estrogenic activity via proliferation (E-SCREEN) and gene expression changes [4] |
| MDA-kb2 cells | Androgen and glucocorticoid receptor screening | Stably transfected with MMTV-luciferase reporter; responsive to androgens and glucocorticoids for screening antagonist/agonist activity [4] |
| GH3 cells | Thyroid hormone disruption assessment | Rat pituitary cell line that proliferates in response to thyroid hormone disruption; used to detect thyroid receptor-mediated effects [4] |
| Recombinant ERα, ERβ, AR | Receptor binding assays | Purified human receptors for high-throughput screening of chemical binding affinity and competitive displacement of natural hormones [4] |
| Yeast Reporter Systems | Transcriptional activation screening | Genetically engineered yeast expressing human hormone receptors and reporter genes; provides cost-effective high-throughput screening [4] |
| ER-EcoScreen | Rapid estrogenicity screening | Chinese hamster ovary cells stably transfected with human ERα and estrogen-responsive luciferase reporter; optimized for sensitivity and throughput [4] |
| Radiolabeled Ligands (³H-estradiol, ³H-testosterone) | Competitive binding assays | High-specific-activity radioligands for precise measurement of receptor binding affinity and kinetics [4] |
| Transthyretin Binding Assay | Thyroid hormone transport disruption | Assesses chemical competition with thyroid hormones for binding to transport proteins; critical for identifying TH disruption mechanisms [4] |
Regulatory Framework and Testing Guidelines
The regulatory landscape for EDCs has evolved significantly, with various international approaches to hazard identification and risk management. In the European Union, criteria for identifying EDCs are established in regulations for biocides (2100/2017), plant protection products (2018/605), and the REACH Regulation [7]. These regulations stipulate that substances posing endocrine disruption risks should not be placed on the market, reflecting the precautionary principle that is fundamental to EU risk governance [7].
The U.S. Environmental Protection Agency has established an endocrine disruptor screening program as mandated by Congress, representing a significant step in improving evaluation and regulation of chemicals with endocrine-disrupting properties [6]. This program employs a two-tiered approach:
Tier 1 Screening:
- Assays to detect interaction with estrogen, androgen, and thyroid systems
- Includes in vitro and short-term in vivo screens
- Designed to identify potential for endocrine activity
Tier 2 Testing:
- Longer-term in vivo studies
- Assesses adverse effects and dose-response relationships
- Focuses on development, reproduction, and growth
The complexity of risk regulation in this field stems from scientific uncertainty, variability in testing methodologies, and challenges in extrapolating from experimental models to human health outcomes [7]. Current research efforts focus on developing new models and tools to better understand how endocrine disruptors work, applying high-throughput assays to identify substances with endocrine-disrupting activity, and identifying new intervention and prevention strategies [2].
Endocrine-disrupting chemicals represent a significant challenge to public health due to their ubiquitous presence in the environment and ability to interfere with hormonal signaling at extremely low concentrations. Understanding the mechanisms of endocrine disruption—from molecular interactions with hormone receptors to systemic health effects—provides the foundation for developing effective regulatory policies and protective measures.
The ten key characteristics of EDCs offer a systematic framework for identifying and evaluating these chemicals, while advancing testing methodologies continue to improve our ability to detect endocrine-disrupting activity. Given the widespread exposure to EDCs through multiple routes including food, air, and dermal absorption, and the growing evidence linking them to serious health outcomes across life stages, continued research and precautionary approaches to chemical management are warranted to reduce population-level exposure and mitigate potential health risks.
Environmental endocrine-disrupting chemicals (EDCs) represent a diverse class of exogenous compounds that interfere with hormonal signaling pathways, posing significant threats to human health. The mechanisms by which these compounds exert their effects are intrinsically linked to their routes of entry into the human body. Understanding the primary exposure triad—ingestion, inhalation, and dermal absorption—is fundamental for researchers, toxicologists, and public health professionals working to assess and mitigate health risks. Exposure to EDCs is widespread and continuous, occurring through multiple pathways simultaneously, which complicates risk assessment and necessitates a thorough understanding of exposure dynamics [9] [10]. This whitepaper synthesizes current scientific evidence on these exposure routes, providing a technical foundation for exposure assessment in research and regulatory contexts.
The concept of "pseudo-persistence" is particularly relevant when considering exposure routes, wherein chemicals that are not inherently persistent in the environment nonetheless maintain a constant presence in human tissues due to continuous exposure from multiple sources and pathways [11]. This phenomenon underscores the importance of evaluating not just the chemical properties of EDCs, but also the human behaviors and environmental contexts that facilitate exposure. With over 85,000 intentionally synthesized chemicals in commerce, and numerous others formed as unintentional byproducts, systematic approaches to exposure science are urgently needed [11].
Defining the Exposure Triad
The human body interfaces with EDCs primarily through three major pathways: ingestion via the gastrointestinal tract, inhalation through the respiratory system, and dermal absorption via the skin. Each route possesses distinct anatomical and physiological characteristics that influence the absorption, distribution, metabolism, and excretion of EDCs. Ingestion represents a major exposure route for EDCs found in food, water, and through hand-to-mouth transfer of contaminated dust [9]. Inhalation introduces airborne EDCs directly into the respiratory system, where they can access the bloodstream or exert local effects in lung tissue [12]. Dermal absorption allows chemicals to penetrate the skin's layers from personal care products, textiles, or environmental media [13].
Real-world exposure scenarios typically involve complex mixtures of EDCs entering through multiple routes simultaneously, creating aggregate exposure that challenges traditional risk assessment paradigms. The timing, duration, and concentration of exposure interact with individual susceptibility factors such as age, genetics, and health status to determine ultimate health outcomes. Critical windows of development—including fetal development, infancy, and puberty—represent periods of heightened susceptibility to EDC exposure through all routes [10] [9].
Table 1: Characteristics of Primary EDC Exposure Routes
| Exposure Route | Primary Sources | Structural Features Enabling Exposure | Key Determinants of Absorption Efficiency |
|---|---|---|---|
| Ingestion | Contaminated food and water, food packaging, hand-to-mouth transfer | High lipid solubility, resistance to digestive enzymes | Gastrointestinal pH, gut microbiota, fat content of food |
| Inhalation | Airborne particles, volatile compounds, dust | Low molecular weight, volatility | Particle size, respiratory rate, alveolar surface area |
| Dermal Absorption | Personal care products, textiles, industrial chemicals | Low molecular weight (<500 Da), lipophilicity | Skin integrity, hydration, vehicle/formulation |
Ingestion as a Primary Exposure Route
The ingestion route constitutes a major pathway for EDC exposure, with dietary intake representing a significant source for many populations. EDCs enter the food chain through multiple mechanisms, including bioaccumulation in animal fats, contamination of agricultural products, and migration from food packaging materials [14]. Plastic monomers and additives such as bisphenol A (BPA) and phthalates are particularly concerning due to their propensity to leach from food containers and packaging into food contents, especially under conditions of heat or prolonged contact [14] [10]. Typical daily intake levels have been documented at 0.1-4 µg/kg body weight for BPA and 1-20 µg/kg body weight for phthalates [14].
Heavy metals and persistent organic pollutants (POPs) also frequently enter the body via ingestion. Cadmium and lead demonstrate exceptional environmental persistence with biological half-lives extending 20-30 years in human tissues, primarily accumulating through contaminated drinking water and food chain bioaccumulation [14]. POPs, including dioxins and polychlorinated biphenyls (PCBs), exhibit exceptional chemical stability allowing environmental persistence exceeding two decades, leading to progressive bioaccumulation through food webs and preferential storage in lipid-rich tissues [14].
Experimental Assessment Methodologies
Biomonitoring approaches for assessing ingestion exposure typically involve analysis of EDCs and their metabolites in biological matrices, with urine and blood being the most common. For biomonitoring of phthalate exposure, researchers typically collect spot urine samples and measure concentrations of monoester metabolites using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). These measurements are often corrected for urinary dilution using creatinine adjustment [9]. For lipophilic compounds that accumulate in adipose tissue, such as PCBs and organochlorine pesticides, serum lipid analysis provides a more appropriate exposure metric than urinary measurements [14].
Dietary exposure assessment often employs duplicate diet studies, where participants consume duplicates of all foods and beverages consumed during a specified period, with subsequent chemical analysis of the composite samples. Migration studies for food contact materials typically involve simulating real-world use conditions by exposing food simulants (e.g., water, 10% ethanol, 3% acetic acid, or olive oil) to packaging materials under controlled time-temperature conditions, followed by chemical analysis of the simulants to quantify leaching [14].
Table 2: Experimental Approaches for Assessing Ingestion Exposure to EDCs
| Methodology | Key Applications | Analytical Techniques | Strengths | Limitations |
|---|---|---|---|---|
| Biomonitoring | Quantifying internal dose from all exposure routes | HPLC-MS/MS, GC-MS | Measures integrated exposure from all sources | Cannot distinguish exposure routes |
| Duplicate Diet Study | Dietary exposure assessment | GC-MS, HPLC-MS/MS | Direct measurement of dietary exposure | Labor-intensive, expensive |
| Migration Testing | Leaching from food contact materials | HPLC-MS/MS, GC-MS | Controlled conditions, reproducible | May not fully replicate real-use conditions |
| Market Basket Survey | Population dietary exposure estimation | GC-MS, HPLC-MS/MS | Representative of food supply | Does not account for individual consumption patterns |
Inhalation Exposure Pathways
Airborne EDCs and Respiratory Uptake
Inhalation exposure to EDCs occurs through airborne particles, volatile organic compounds, and semi-volatile compounds that partition to dust particles. Phthalates, which are commonly used as plasticizers, demonstrate significant inhalation exposure potential due to their presence in indoor air and dust [12]. A systematic review and meta-analysis found that phthalates in dust samples were significantly associated with asthma onset in children and adolescents (OR: 1.21, 95% CI: 1.02-1.44) [12]. Polybrominated diphenyl ethers (PBDEs) used as flame retardants exhibit remarkable biological persistence, with half-lives extending 3-7 years and high octanol-water partition coefficients (log Kow = 6.5-8.4), facilitating extensive bioaccumulation through inhalation of household dust [14].
Recent research has expanded to include respiratory health outcomes beyond asthma. A 2025 study analyzing NHANES data revealed that exposure to EDC mixtures was associated with preserved ratio impaired spirometry (PRISm), a precursor to chronic obstructive pulmonary disease (COPD) [15]. Weighted quantile sum regression demonstrated that each index rise in the EDC-mixture increased the odds of PRISm by 63% (OR=1.63, 95% CI: 1.25-2.13), with mono-isobutyl phthalate (MIBP) identified as a primary driver of this effect (OR=2.29, 95% CI: 1.71-3.07) [15].
Methodologies for Air Sampling and Analysis
Assessment of inhalation exposure requires specialized air sampling techniques that vary based on the physical-chemical properties of target EDCs. For semi-volatile organic compounds (SVOCs) like phthalates and PBDEs, settled dust sampling is commonly employed using standardized vacuum sampling protocols with predetermined sampling areas and nozzle sizes [12]. Dust samples are typically sieved to a specific particle size (e.g., <100 μm) before extraction and analysis via gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Active air sampling methods utilize pumps to draw air through collection media, such as polyurethane foam (PUF) plugs for volatile compounds or quartz fiber filters for particle-bound compounds. These approaches allow for determination of airborne concentrations but require specialized equipment and may not capture temporal variability in exposure. Passive air samplers, including PUF passive samplers or silicone wristbands, provide time-integrated measurements that can be deployed in personal exposure assessment studies [12].
Dermal Absorption Mechanisms
Skin Penetration Dynamics
Dermal absorption constitutes a significant exposure pathway for EDCs present in personal care products, cosmetics, and textiles. The skin's structure presents a complex barrier with the stratum corneum serving as the primary rate-limiting layer for chemical penetration. Key chemical properties influencing dermal absorption include molecular weight (with compounds <500 Da penetrating more readily), lipophilicity (optimal log Kow 1-3), and hydrogen bonding potential [16]. The dermal route is particularly relevant for EDCs such as phthalates, parabens, triclosan, and ultraviolet filters found in cosmetics and personal care products.
A 2025 systematic review of cosmetic ingredients identified 27 chemicals categorized as having "indications for ED properties" out of 890 cosmetic ingredients reviewed, highlighting the prevalence of potential EDCs in products that directly contact skin [16]. The study developed a workflow to screen cosmetic ingredients against known EDC lists from regulatory agencies and scientific sources, providing a methodology for prioritizing chemicals for further safety assessment. For one of these chemicals, geraniol, aggregate exposure was modeled using PACEMweb software and compared to the lowest observed adverse effect level from toxicological studies, demonstrating an approach for evaluating endocrine-related health risks from dermal exposure [16].
Experimental Models for Dermal Absorption
In vitro dermal absorption testing typically employs human or animal skin membranes mounted in Franz diffusion cells, which maintain physiological temperature and humidity conditions. The skin membrane separates a donor chamber (containing the test material) from a receptor chamber (containing collection fluid), allowing quantification of chemical permeation over time [16]. Excised human skin from surgical procedures represents the gold standard, while reconstructed human skin models (e.g., EpiDerm, EpiSkin) offer alternatives that avoid animal use.
In vivo studies, while less common due to ethical concerns and regulatory restrictions, provide important validation data for in vitro models. Human volunteer studies sometimes use topical application of stable isotope-labeled EDCs to distinguish applied chemicals from background exposure, with subsequent measurement of metabolites in urine or blood to determine systemic absorption [16]. Alternative approaches include the use of silicone wristbands or other passive samplers worn on the skin to assess potential dermal exposure, though these measure available concentration rather than actual absorption [13].
Integrated Exposure Assessment and Research Gaps
Aggregate Exposure Assessment
Comprehensive EDC risk assessment requires integration of exposure across all three routes—ingestion, inhalation, and dermal absorption—to determine total body burden. Aggregate exposure assessment presents substantial methodological challenges due to differences in measurement approaches, timing of exposure, and metabolic fate across routes. Biomonitoring data, which measure the internal concentration of EDCs or their metabolites, provide integrated measures of exposure from all routes but cannot distinguish the contribution of each pathway [17].
Computational approaches for aggregate exposure assessment include probabilistic models that combine exposure factors, such as those implemented in the PACEMweb software used for cosmetic safety assessment [16]. These models integrate product composition data, usage patterns, and absorption parameters to estimate total systemic exposure. For EDCs with multiple exposure sources, such as phthalates, studies have demonstrated that exposure routes can be complementary, with no single source dominating total exposure, necessitating comprehensive assessment approaches [17].
Key Research Gaps and Methodological Challenges
Substantial knowledge gaps persist in understanding the relative contribution of different exposure routes to total EDC body burden. Complex mixture effects remain poorly understood, as most toxicological studies focus on single chemicals despite real-world exposure involving simultaneous contact with multiple compounds through multiple routes [14]. The "cocktail effect" of EDC mixtures may produce synergistic or additive effects that are not predictable from single-chemical studies.
Non-monotonic dose responses (NMDRs) present another significant challenge, particularly for EDCs that can produce more pronounced effects at low doses than at high doses [9]. This phenomenon complicates the establishment of threshold values and safe exposure limits. Additionally, critical windows of susceptibility—such as fetal development, infancy, and puberty—may exhibit enhanced sensitivity to EDCs, but route-specific exposure data during these periods are limited [5] [10].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Materials and Methods for EDC Exposure Assessment
| Tool/Category | Specific Examples | Primary Research Application | Technical Considerations |
|---|---|---|---|
| Analytical Standards | Deuterated BPA, carbon-13 labeled phthalates, native POP standards | Instrument calibration, isotope dilution quantification, recovery calculations | Purity verification, stability during storage, preparation of mixed stock solutions |
| Biological Matrices | Pooled human urine, serum, synthetic urine, artificial sweat | Quality control materials, method development, matrix effect studies | Homogeneity, stability, commutability with native samples |
| Sample Collection Media | Silanized glass vials, PUF plugs, quartz filters, silicone wristbands | Environmental and biological sample collection, storage, and transport | Blank levels, recovery efficiency, sample stability |
| Chromatography Columns | C18 reverse-phase, HILIC, phenyl-modified stationary phases | Separation of EDCs and metabolites from complex matrices | Column selectivity, peak shape, retention time stability |
| Mass Spectrometry | Triple quadrupole MS, high-resolution accurate mass (HRAM) instruments | Target quantification and suspect screening | Sensitivity, selectivity, dynamic range, mass accuracy |
The primary exposure triad of ingestion, inhalation, and dermal absorption represents fundamental pathways through which EDCs enter the human body and disrupt endocrine function. Each route involves distinct exposure sources, physiological barriers, and methodological approaches for assessment. Current evidence demonstrates that real-world exposure typically occurs through multiple routes simultaneously, resulting in cumulative body burdens that are challenging to quantify and link to specific health outcomes. Advancements in exposure science, including improved biomonitoring techniques, computational modeling, and novel sampling approaches, continue to enhance our understanding of these complex exposure pathways. Future research priorities should include development of standardized methods for aggregate exposure assessment, investigation of mixture effects across exposure routes, and elucidation of critical windows of susceptibility throughout the lifespan.
This technical guide provides an in-depth analysis of three major classes of chemicals of concern—Plasticizers, Heavy Metals, and Persistent Organic Pollutants (POPs)—within the context of Endocrine Disrupting Chemical (EDC) exposure research. These substances represent significant environmental health challenges due to their persistence, bioavailability, and ability to interfere with hormonal systems through multiple exposure routes including food, air, and dermal absorption [8] [18]. Growing evidence links EDC exposure to diverse adverse health outcomes including reproductive impairment, neurodevelopmental disorders, metabolic dysfunction, and cancer, driving urgent need for comprehensive risk assessment frameworks and mitigation strategies [8].
The following sections detail the properties, exposure pathways, health impacts, and analytical methods for each chemical class, with specific emphasis on their roles as endocrine disruptors. Technical protocols and research tools are provided to support scientific investigation and drug development efforts aimed at understanding and counteracting their toxicological effects.
Plasticizers
Definition and Key Applications
Plasticizers are additive chemicals used primarily to increase the flexibility, durability, and workability of polymeric materials, especially polyvinyl chloride (PVC) [19]. They function by embedding themselves between polymer chains, reducing secondary molecular bonds and decreasing glass transition temperature, thereby making rigid materials more pliable [19]. Global plasticizer market volume is projected to grow from $17 billion in 2022 to $22.5 billion by 2027, with non-phthalate alternatives representing an increasing share (approximately 35% in 2017, expected to reach 40%) [20].
Table 1: Major Plasticizer Classes and Applications
| Chemical Class | Major Compounds | Primary Applications | Key Properties |
|---|---|---|---|
| Phthalates | DEHP, DINP, DIDP, DBP | PVC products, vinyl flooring, cables, medical devices [19] | Good plasticizing efficiency, low cost, durability [19] |
| Non-Phthalate Plasticizers (NPPs) | DEHT, DINCH, ATBC, ESBO | Food packaging, medical devices, children's toys, cosmetics [21] [20] | Perceived as safer alternatives; varying efficacy [21] |
| Adipates | DEHA, DINA, DiDA | Food packaging films, synthetic leather [21] | Improved low-temperature flexibility [21] |
| Trimellitates | TOTM | High-temperature wire and cable insulation [19] | High temperature resistance, lower volatility [19] |
| Citrates | ATBC | Food contact materials, medical devices, cosmetics [20] | Low toxicity, biodegradable [20] |
| Polyesters | Various | Require high permanence applications [19] | Low migration, high molecular weight [19] |
Plasticizers as Endocrine Disruptors
Substantial evidence identifies numerous plasticizers as endocrine disrupting chemicals (EDCs) that interfere with hormonal signaling pathways. Multiple epidemiological studies demonstrate consistent associations between plasticizer exposure and adverse reproductive outcomes including impaired semen quality, decreased ovarian reserve, infertility, polycystic ovary syndrome (PCOS), and altered hormone levels [18]. The mechanisms of disruption include:
- Estrogen and Androgen Receptor Interactions: Many plasticizers mimic or block natural hormones, particularly estrogen and androgen, altering receptor activation and downstream gene expression [18].
- Steroidogenesis Interference: Plasticizers can disrupt the synthesis and metabolism of endogenous steroid hormones [21].
- Nuclear Receptor Activation: Compounds including DEHP, DINP, and DINCH can activate peroxisome proliferator-activated receptors (PPARs) which regulate lipid metabolism and adipogenesis [20].
Emerging research indicates that some non-phthalate alternatives initially marketed as safer may present similar endocrine-disrupting concerns, representing cases of "regrettable substitution" [20]. For example, alternative plasticizers such as acetyl tributyl citrate (ATBC), diisononyl cyclohexane-1,2-dicarboxylate (DINCH), and tris-2-ethylhexyl phosphate (TEHP) show potential endocrine disrupting properties [20].
Exposure Routes and Experimental Assessment
Human exposure to plasticizers occurs through multiple pathways, with the relative contribution of each route varying by compound properties and population-specific factors:
- Food Ingestion: Primary exposure route for many plasticizers, especially through migration from food packaging and processing equipment [21]. Lipid-rich foods particularly accumulate lipophilic plasticizers.
- Dermal Absorption: Direct contact with personal care products, cosmetics, and vinyl materials containing plasticizers [21].
- Inhalation: Indoor air and dust containing volatilized or particulate-bound plasticizers, especially in environments with PVC flooring or furnishings [20].
- Parenteral Exposure: Direct introduction into bloodstream via medical devices such as PVC intravenous tubing and blood bags [19].
Table 2: Analytical Methods for Plasticizer Assessment
| Matrix | Sample Preparation | Analytical Technique | Key Parameters |
|---|---|---|---|
| Urine | Enzymatic deconjugation, solid-phase extraction, dilution [18] | LC-MS/MS | Secondary metabolites (e.g., monoesters for phthalates, oxidative metabolites for DINCH) |
| Dust | Accelerated solvent extraction, gel permeation chromatography cleanup [20] | GC-MS | Multiple plasticizer concentrations; source attribution |
| Food Simulants | Solvent extraction, membrane filtration | HPLC-UV/FLD | Migration rates under standardized conditions |
| Serum | Protein precipitation, liquid-liquid extraction | HPLC-MS/MS | Parent compounds and biomarkers of effect |
| Indoor Air | Active sampling (sorbent tubes), passive air samplers | GC-MS | Gas-phase and particle-phase concentrations |
Experimental Protocol: Biomonitoring of Plasticizer Metabolites
- Sample Collection: Collect spot or first-morning void urine samples in pre-cleaned polypropylene containers; store at -80°C until analysis.
- Sample Preparation: Thaw samples overnight at 4°C. Mix by vortexing. Aliquot 1 mL urine into reaction vials. Add internal standard mixture (deuterated analogs).
- Enzymatic Hydrolysis: Adjust pH to 6.5 with ammonium acetate buffer. Add β-glucuronidase/arylsulfatase preparation. Incubate at 37°C for 90 minutes.
- Solid-Phase Extraction: Load hydrolysate onto preconditioned Oasis HLB cartridges. Wash with 5% methanol in water. Elute with methanol.
- Analysis: Concentrate eluent under nitrogen stream. Reconstitute in mobile phase. Analyze by LC-MS/MS using multiple reaction monitoring (MRM).
- Quality Control: Include method blanks, quality control pools, and standard reference materials with each batch [18].
Research Reagent Solutions
Table 3: Essential Research Reagents for Plasticizer Analysis
| Reagent/Material | Function | Application Context |
|---|---|---|
| Stable Isotope-Labeled Internal Standards (e.g., D4-DEHP, 13C-BPA) | Quantification correction for recovery and matrix effects | LC-MS/MS biomonitoring |
| β-Glucuronidase/Arylsulfatase (Helix pomatia) | Enzymatic deconjugation of phase II metabolites | Urine sample preparation prior to extraction |
| Oasis HLB Solid-Phase Extraction Cartridges | Extraction and cleanup of analytes from biological matrices | Sample preparation for urine, serum |
| C18 Reverse Phase Chromatography Columns | Separation of analytes by hydrophobicity | LC-MS/MS analysis |
| Certified Reference Materials (NIST SRM 3672, 3673) | Method validation and quality assurance | Organic contaminants in dust and soils |
| Molecularly Imprinted Polymers | Selective extraction of target analytes | Sample clean-up for complex matrices |
| Recombinant Nuclear Receptor Assays (ERα, AR, PPARγ) | Assessment of endocrine disruption potential | High-throughput screening of plasticizers |
Heavy Metals
Environmental Presence and Health Risks
Heavy metals represent a significant class of inorganic contaminants with well-established endocrine disrupting properties. Agricultural soil contamination has become increasingly severe due to urbanization and industrialization, with heavy metals permeating soil ecosystems through atmospheric deposition, surface runoff, and subsurface migration [22]. These elements pose significant ecological risks due to their environmental persistence, bioaccumulation potential, and toxicity [22].
Table 4: Heavy Metals of Concern: Sources and Health Effects
| Heavy Metal | Major Anthropogenic Sources | Primary Health Effects | Endocrine Disruption Mechanisms |
|---|---|---|---|
| Arsenic (As) | Pesticide residues, industrial waste, mining [22] | Skin lesions, cardiovascular disease, cancer [23] | Glucocorticoid receptor disruption, steroidogenesis interference [8] |
| Cadmium (Cd) | Phosphate fertilizers, battery manufacturing, metal plating [22] | Renal dysfunction, osteoporosis, cancer [22] | Estrogen receptor activation, progesterone receptor suppression |
| Lead (Pb) | Leaded gasoline, paints, electronic waste, mining [23] | Neurodevelopmental deficits, anemia, hypertension [23] | Hypothalamic-pituitary axis disruption, growth hormone alteration |
| Chromium (Cr) | Tanneries, textile manufacturing, metal plating [23] | Allergic dermatitis, lung cancer, renal damage [23] | Oxidative stress, hormone receptor modification |
| Nickel (Ni) | Metal alloy production, combustion of fossil fuels [23] | Contact dermatitis, lung fibrosis, nasal cancer [23] | Hypoxia signaling pathway activation |
| Mercury (Hg) | Coal combustion, gold mining, dental amalgams [8] | Neurological impairment, renal damage, developmental toxicity [8] | Thyroid hormone disruption, estrogenic effects |
Exposure Assessment and Risk Quantification
Heavy metal exposure occurs through ingestion of contaminated food and water, inhalation of particulate matter, and dermal contact with contaminated media [23]. Advanced risk assessment approaches integrate multiple exposure pathways using probabilistic methods:
Table 5: Health Risk Assessment Indices for Heavy Metals
| Assessment Index | Calculation Formula | Interpretation |
|---|---|---|
| Geo-accumulation Index (Igeo) | Igeo = log₂(Cn/1.5Bn) where Cn is measured concentration, Bn is background concentration [22] | Igeo ≤ 0: unpolluted; Igeo > 5: extremely polluted |
| Contamination Factor (CF) | CF = Cmetal/Cbackground | CF < 1: low contamination; CF ≥ 6: very high contamination |
| Pollution Load Index (PLI) | PLI = (CF1 × CF2 × ... × CFn)^1/n | PLI > 1 indicates deterioration of site quality |
| Hazard Quotient (HQ) | HQ = CDI/RfD where CDI is chronic daily intake, RfD is reference dose [23] | HQ < 1: unlikely adverse effects; HQ ≥ 1: potential adverse effects |
| Hazard Index (HI) | HI = ΣHQingestion + ΣHQinhalation + ΣHQdermal [23] | HI < 1: safe level; HI ≥ 1: potential non-carcinogenic risk |
| Carcinogenic Risk (CR) | CR = CDI × SF where SF is slope factor | CR < 10⁻⁶: negligible risk; CR > 10⁻⁴: unacceptable risk |
Experimental Protocol: Soil Heavy Metal Analysis and Risk Assessment
- Sample Collection: Collect surface (0-20 cm) and deep (150-200 cm) soil samples using stainless steel tools at predetermined grid points. Record GPS coordinates.
- Sample Preparation: Air-dry samples at room temperature, remove visible gravel and plant roots. Grind and homogenize using agate mortar, sieve through 2-mm nylon mesh.
- Acid Digestion: Weigh 0.5 g soil sample into Teflon digestion vessel. Add 9 mL HNO₃, 3 mL HCl, and 2 mL HF. Digest using microwave-assisted system with temperature ramp to 180°C maintained for 15 minutes.
- Analysis: Cool digested samples, transfer to volumetric flasks, dilute to 50 mL with deionized water. Analyze using ICP-OES with appropriate quality controls (blanks, duplicates, certified reference materials BCR-667).
- Spatial Analysis: Input georeferenced concentration data into GIS software (QGIS). Apply inverse distance weighting (IDW) interpolation to create contamination distribution maps.
- Risk Calculation: Apply Monte Carlo simulation (10,000 iterations) to account for parameter uncertainty in exposure factors. Calculate Hazard Quotients (HQ) and Hazard Index (HI) for different age groups via oral ingestion, inhalation, and dermal contact pathways [22] [23].
Research Reagent Solutions
Table 6: Essential Research Reagents for Heavy Metal Analysis
| Reagent/Material | Function | Application Context |
|---|---|---|
| High-Purity Acids (HNO₃, HCl, HF) | Sample digestion and dissolution | Microwave-assisted acid digestion of environmental samples |
| Certified Reference Materials (NIST SRM 2709, 2710, BCR-667) | Quality assurance and method validation | Analytical accuracy verification for soil/sediment analysis |
| ICP-MS/MS Tuning Solutions | Instrument calibration and optimization | Sensitivity and oxide/carbon interference minimization |
| Single-Element Stock Standards (1000 mg/L) | Calibration curve preparation | Quantitative analysis by ICP-OES/ICP-MS |
| Chelating Resins (Chelex-100, IMAC) | Preconcentration and matrix separation | Trace metal analysis in environmental waters |
| Modified DGT (Diffusive Gradients in Thin Films) | In-situ measurement of bioavailable metals | Passive sampling in waters and sediments |
| CRMs for Biological Monitoring (Seruon, Blood, Urine) | Quality control for biomonitoring | Human exposure assessment |
Persistent Organic Pollutants (POPs)
Definition and Regulatory Framework
Persistent Organic Pollutants (POPs) are toxic chemical compounds that resist natural degradation processes, persist in the environment for extended periods, bioaccumulate in living organisms, and biomagnify through food chains [24] [25]. The Stockholm Convention on Persistent Organic Pollutants, adopted in 2001, is a global treaty to protect human health and the environment from POPs, with over 180 participating countries committing to control and reduce these substances [24] [25].
POPs include intentionally produced chemicals such as industrial compounds and pesticides, as well as unintentional byproducts formed during combustion and industrial processes [24]. Key characteristics include:
- Persistence: Resistance to photolytic, chemical, and biological degradation (half-lives exceeding 6 months)
- Bioaccumulation: Bioconcentration factors > 5,000 in aquatic species
- Long-Range Transport: Capable of traveling thousands of kilometers from emission sources
- Toxicity: Adverse effects on human health and wildlife at low concentrations
The "Dirty Dozen" and Beyond
The original "Dirty Dozen" POPs identified by the Stockholm Convention include [24]:
Table 7: Initial POPs Listed under Stockholm Convention
| POP Category | Specific Compounds | Historical Uses |
|---|---|---|
| Pesticides | Aldrin, Chlordane, DDT, Dieldrin, Endrin, Heptachlor, Mirex, Toxaphene | Agricultural pest control, vector management |
| Industrial Chemicals | Hexachlorobenzene, Polychlorinated Biphenyls (PCBs) | Electrical equipment, solvents, fungicides |
| Byproducts | Polychlorinated dibenzo-p-dioxins (dioxins), Polychlorinated dibenzofurans (furans) | Combustion processes, chemical manufacturing |
While many legacy POPs are now banned or restricted in most countries, their persistence ensures continued environmental presence and health impacts. DDT, for example, remains in limited use for malaria control in some regions despite widespread bans, highlighting the challenge of balancing public health benefits against environmental contamination [24].
Endocrine Disruption Mechanisms
POPs exert endocrine disrupting effects through multiple mechanisms that interfere with hormonal signaling pathways:
- Receptor-Mediated Effects: Many POPs interact directly with nuclear hormone receptors, particularly estrogen receptors (ER), androgen receptors (AR), thyroid hormone receptors (TR), and aryl hydrocarbon receptor (AhR) [25] [18].
- Hormone Synthesis Alteration: POPs can interfere with steroidogenic enzymes including cytochrome P450 complexes, altering the synthesis and metabolism of endogenous hormones [8].
- Hormone Transport Disruption: Some POPs compete with thyroid hormones for binding to transport proteins such as transthyretin, affecting hormone distribution and bioavailability [18].
- Cellular Signaling Interference: POPs can disrupt post-receptor signaling pathways including calcium signaling and protein kinase cascades [8].
Experimental Protocol: POPs Analysis in Biological Samples
- Sample Collection and Storage: Collect adipose tissue, serum, or breast milk in pre-cleaned glass containers. Store at -20°C or lower to prevent degradation.
- Lipid Extraction: Homogenize sample with anhydrous sodium sulfate. Extract lipids using accelerated solvent extraction (ASE) with dichloromethane:hexane (1:1, v/v) at 100°C and 1500 psi.
- Lipid Removal and Cleanup: Determine lipid content gravimetrically. Remove lipids using gel permeation chromatography (GPC) or sulfuric acid treatment.
- Fractionation: Separate compound classes using silica gel column chromatography with sequential elution using hexane, hexane:dichloromethane, and dichloromethane:methanol.
- Instrumental Analysis: Analyze fractions using gas chromatography coupled to high-resolution mass spectrometry (GC-HRMS) with electron impact ionization.
- Quality Assurance: Include procedural blanks, matrix spikes, and certified reference materials (NIST SRM 1957, 1947) with each batch. Use isotope dilution quantification with 13C-labeled internal standards [24].
Research Reagent Solutions
Table 8: Essential Research Reagents for POPs Analysis
| Reagent/Material | Function | Application Context |
|---|---|---|
| 13C/2H-Labeled Internal Standards | Quantification correction and recovery monitoring | Isotope dilution mass spectrometry |
| Silica Gel, Alumina, Florisil | Adsorbents for chromatographic cleanup | Sample preparation and fractionation |
| Gel Permeation Chromatography (GPC) | Lipid removal from biological extracts | Sample cleanup prior to GC analysis |
| HRGC-HRMS Systems | High-resolution separation and detection | Congener-specific POPs analysis |
| CALUX Bioassay Kits (Chemically Activated LUciferase eXpression) | Bioanalytical screening for dioxin-like activity | High-throughput toxicity screening |
| Certified Reference Materials (NIST, WMF, BCR) | Method validation and quality control | Analytical accuracy verification |
| Passive Air Samplers (PUF disks, XAD resins) | Monitoring atmospheric POPs | Long-term spatial and temporal trend studies |
Plasticizers, heavy metals, and persistent organic pollutants represent three distinct but interconnected classes of endocrine disrupting chemicals with significant implications for human and environmental health. Despite differing in chemical structure and applications, they share common features including environmental persistence, bioaccumulation potential, and the ability to disrupt hormonal systems at low exposure levels.
Research gaps remain in understanding the full scope of health impacts, particularly from chronic low-dose exposures and complex mixtures. The phenomenon of "regrettable substitution"—replacing regulated chemicals with structurally similar alternatives that may pose comparable risks—highlights the need for more comprehensive chemical safety assessment frameworks [21] [20]. Advanced analytical techniques, biomonitoring programs, and computational toxicology approaches will be essential for identifying emerging concerns and developing effective risk management strategies.
Future research should prioritize longitudinal studies to assess cumulative effects, elucidate mechanisms of action across different life stages, and identify susceptible populations. Integration of novel approach methodologies including high-throughput screening, organ-on-a-chip systems, and adverse outcome pathways will accelerate the identification of hazardous properties and support evidence-based decision making for chemical regulation and public health protection.
Environmental endocrine-disrupting chemicals (EDCs) represent a diverse class of synthetic and naturally occurring compounds that interfere with the normal function of the endocrine system. The molecular mechanisms through which these chemicals exert their effects are complex and multifaceted, primarily involving receptor interference, oxidative stress induction, and epigenetic modifications [14] [26]. Understanding these mechanisms is crucial for assessing the full scope of health risks posed by EDCs, which enter the human body through food, air, and skin absorption [14] [27].
Research demonstrates that EDCs disrupt hormonal homeostasis through direct and indirect pathways, leading to adverse health outcomes including reproductive dysfunction, metabolic disorders, neurodevelopmental issues, and hormone-sensitive cancers [28] [26]. This technical review examines the core molecular mechanisms of EDC action, provides quantitative data on exposure thresholds and effects, outlines experimental methodologies for mechanistic studies, and visualizes key signaling pathways disrupted by these ubiquitous environmental contaminants.
Molecular Mechanisms of Action
Receptor Interference
EDCs primarily disrupt endocrine function by directly interfering with hormone receptor signaling pathways. These chemicals can mimic natural hormones, antagonize their actions, or alter receptor expression and function [14] [29].
Nuclear Receptor Interactions: Many EDCs exert their effects through binding to nuclear hormone receptors, particularly estrogen receptors (ERs), androgen receptors (ARs), progesterone receptors, thyroid receptors, and retinoid receptors [29] [26]. Bisphenol A (BPA) demonstrates high-affinity binding to estrogen receptors, fundamentally altering hormonal balance at typical daily intake levels of 0.1-4 µg/kg body weight [14] [27]. Phthalates, particularly di(2-ethylhexyl) phthalate (DEHP), interfere with androgen receptor signaling and are routinely detected in seminal plasma at concentrations of 0.77-1.85 μg/mL, with documented associations to reduced sperm concentration and motility [14] [27].
Non-Monotonic Dose Responses: EDCs frequently exhibit non-monotonic dose-response (NMDR) relationships, where low-dose chronic exposure may produce more pronounced biological effects than acute high-dose exposure [14] [30]. Computational modeling suggests that interference with systemic negative feedback regulation in endocrine axes serves as a potential mechanism for these counterintuitive dose-response patterns [30]. The NMDR phenomenon complicates traditional toxicological risk assessment, which typically assumes monotonic dose-response relationships.
Table 1: Receptor-Mediated Mechanisms of Select EDCs
| EDC Category | Specific Compounds | Primary Receptors Targeted | Affinity/EC50 | Cellular Consequences |
|---|---|---|---|---|
| Plasticizers | Bisphenol A (BPA) | ERα, ERβ, GPR30 | High affinity for ERβ [27] | Altered estrogen signaling, cell proliferation |
| Phthalates | DEHP, DBP, DEP | Androgen Receptor, PPARγ | Seminal concentrations: 0.77-1.85 μg/mL [27] | Reduced sperm quality, testosterone suppression |
| Persistent Organic Pollutants | PCBs, Dioxins | Aryl hydrocarbon Receptor, ER | Toxic equivalence factors: 0.0001-1 [14] | Disrupted steroidogenesis, oxidative stress |
| Heavy Metals | Cadmium, Lead | Estrogen Receptor, Glucocorticoid Receptor | Blood lead >10 μg/dL causes sperm DNA damage [14] | Receptor activation, hormone mimicry |
Oxidative Stress Induction
Oxidative stress represents a central mechanism in EDC toxicity, occurring when the production of reactive oxygen species (ROS) overwhelms cellular antioxidant defenses [14] [31].
Reactive Oxygen Species Generation: Multiple EDCs induce excessive ROS production through various pathways. Titanium dioxide nanoparticles trigger ROS generation resulting in sperm membrane damage with an ED50 of 150 mg/kg [14]. Heavy metals including cadmium and lead promote oxidative stress through Fenton chemistry and depletion of glutathione, a key cellular antioxidant [14]. Phthalates like DEHP undergo metabolic activation to generate free radicals that oxidize cellular macromolecules [32].
Cellular Consequences: Oxidative damage from EDCs affects lipids, proteins, and DNA, leading to membrane disruption, enzyme inactivation, and mitochondrial dysfunction [14] [31]. In the cardiovascular system, EDCs have been shown to promote atherosclerosis through ROS-mediated endothelial dysfunction, impaired nitric oxide production, and oxidative modification of LDL cholesterol [31]. In male reproduction, oxidative stress damages sperm membrane integrity and DNA, compromising fertilizing potential and embryo development [14].
Mediating Role in Disease Processes: Recent evidence identifies oxidative stress as a critical mediator linking EDC exposure to various disease outcomes. A 2025 cross-sectional study demonstrated that oxidative stress biomarkers (bilirubin and iron) mediated the relationship between EDC mixtures and phenotypic aging acceleration, with mediation proportions of -24.86% and -21.06%, respectively [33].
Table 2: Oxidative Stress Biomarkers in EDC Research
| Biomarker Category | Specific Markers | Detection Methods | Significance in EDC Research |
|---|---|---|---|
| Lipid Peroxidation | MDA, 4-HNE, 8-iso-PGF2α | HPLC, ELISA, GC-MS | Indicates membrane damage, atherosclerosis risk [31] |
| DNA Oxidation | 8-OHdG, Oxoguanine glycosylase | LC-MS, Immunoassays | Genotoxic effects, cancer risk assessment |
| Protein Carbonyls | Carbonylated proteins | DNPH assay, Western blot | Cellular dysfunction, enzyme inactivation |
| Antioxidant Enzymes | SOD, Catalase, GPx | Activity assays, ELISA | Compensatory response, oxidative burden |
| Non-enzymatic Antioxidants | Glutathione, Bilirubin | Colorimetric assays, HPLC | Mediating role in EDC-aging relationship [33] |
Epigenetic Modifications
Epigenetic mechanisms represent a crucial pathway through which EDCs exert long-lasting health effects, including transgenerational inheritance of disease susceptibility [14] [32].
DNA Methylation Changes: EDC exposure induces alterations in DNA methylation patterns, particularly at imprinted genes and regulatory elements of hormone-responsive genes [32]. Phthalate exposure has been associated with hypomethylation of the H19 locus, an imprinted gene involved in growth regulation [26]. These changes can persist long after exposure cessation and may be transmitted to subsequent generations, as demonstrated in animal studies [14] [32].
Histone Modifications: EDCs alter post-translational modifications of histone proteins, including acetylation, methylation, phosphorylation, and ubiquitination [32] [26]. These modifications change chromatin accessibility and gene expression patterns without altering the underlying DNA sequence. For example, paternal exposure to EDCs has been linked to histone modifications in sperm that may influence offspring health outcomes [26].
Noncoding RNA Expression: MicroRNAs and other noncoding RNAs are differentially expressed following EDC exposure and contribute to their toxic effects by regulating gene expression at the post-transcriptional level [32]. Phthalates have been shown to induce organ-specific changes in miRNA expression profiles, potentially contributing to disease pathogenesis in hormone-sensitive tissues [32].
Table 3: Epigenetic Alterations Induced by EDCs
| Epigenetic Mechanism | EDCs with Documented Effects | Specific Genes/Regions Altered | Functional Consequences |
|---|---|---|---|
| DNA Methylation | Phthalates, BPA, PCBs | H19 locus hypomethylation [26] | Growth dysregulation, infertility |
| Histone Modification | BPA, Phthalates | Histone acetylation/methylation changes [26] | Altered chromatin structure, gene expression |
| Noncoding RNA | DEHP, BPA, Heavy Metals | miRNA expression profiles [32] | Organ-specific toxicity, disease risk |
| Transgenerational Epigenetic Inheritance | Vinclozolin, Phthalates | Sperm epigenome alterations [32] | Multi-generational reproductive effects |
Experimental Approaches and Methodologies
Receptor Binding Assays
Competitive Binding Studies: Experimental protocols for assessing EDC receptor interactions typically employ competitive binding assays using tritiated or fluorescently-labeled natural ligands. Cell-free systems expressing purified hormone receptors are incubated with radiolabeled reference ligands (e.g., [3H]-estradiol for ER binding) in the presence of increasing concentrations of EDCs. Non-specific binding is determined by parallel incubations with a large excess of unlabeled ligand. Following incubation, bound and free ligands are separated by charcoal-dextran adsorption, filtration, or size-exclusion chromatography, and radioactivity or fluorescence is quantified [29].
Transcriptional Activation Assays: Reporter gene assays in cell culture models provide functional assessment of EDC effects on receptor signaling. Cells are transfected with expression vectors for specific nuclear receptors and corresponding reporter constructs containing receptor-responsive elements upstream of luciferase or other easily quantifiable genes. After exposure to EDCs, reporter activity is measured to determine whether the test compound acts as an agonist, antagonist, or mixed function modulator [29].
Structural Studies: X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy reveal atomic-level interactions between EDCs and hormone receptors, identifying key binding pocket residues and conformational changes that underlie receptor activation or inhibition [29].
Oxidative Stress Assessment
ROS Detection Methods: Intracellular ROS generation following EDC exposure is typically quantified using fluorescent probes such as 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA), dihydroethidium (DHE) for superoxide, and MitoSOX Red for mitochondrial superoxide. Flow cytometry, fluorescence microscopy, and microplate readers are used for detection and quantification [33] [31].
Biomarker Analysis: Oxidative damage biomarkers are measured using various techniques. Lipid peroxidation products (MDA, 4-HNE) are quantified by HPLC with fluorescence detection or ELISA. DNA oxidation marker 8-OHdG is measured by LC-MS/MS or immunoassays. Protein carbonyl content is determined by derivatization with dinitrophenylhydrazine (DNPH) followed by spectrophotometric detection or Western blotting [33] [31].
Antioxidant Capacity Assays: Cellular antioxidant status is evaluated by measuring antioxidant enzyme activities (SOD, catalase, GPx) through spectrophotometric methods and quantifying non-enzymatic antioxidants (glutathione, bilirubin) using colorimetric, fluorometric, or HPLC-based approaches [33].
Epigenetic Analysis
DNA Methylation Profiling: Genome-wide DNA methylation patterns are assessed using bisulfite conversion-based methods followed by sequencing (Whole Genome Bisulfite Sequencing, Reduced Representation Bisulfite Sequencing) or array-based platforms (Infinium MethylationEPIC BeadChip). Locus-specific methylation is analyzed by bisulfite pyrosequencing or methylation-specific PCR [32].
Histone Modification Analysis: Chromatin immunoprecipitation (ChIP) assays using antibodies specific to modified histones (e.g., H3K27ac, H3K4me3) followed by qPCR (ChIP-qPCR) or sequencing (ChIP-seq) enable genome-wide mapping of histone modifications. Western blotting with modification-specific antibodies provides global assessment of histone mark levels [32].
Noncoding RNA Expression Profiling: Next-generation sequencing of small RNA libraries identifies differential expression of miRNAs and other noncoding RNAs following EDC exposure. RT-qPCR with stem-loop primers enables validation and quantification of specific miRNAs of interest [32].
Pathway Visualization
Diagram 1: Integrated Molecular Mechanisms of EDC Action. This pathway illustrates how EDC exposure through various routes triggers three primary molecular mechanisms that converge to produce adverse health outcomes.
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Research Reagents for EDC Mechanism Studies
| Reagent Category | Specific Examples | Research Application | Technical Considerations |
|---|---|---|---|
| Reporter Plasmids | ER/AR-responsive luciferase constructs | Receptor activation profiling | Vector backbone affects sensitivity; include controls for cytotoxicity |
| ROS Detection Probes | DCFH-DA, MitoSOX Red, DHE | Oxidative stress measurement | Select appropriate probe for specific ROS; consider compartmentalization |
| Epigenetic Inhibitors | 5-azacytidine (DNMT inhibitor), TSA (HDAC inhibitor) | Mechanistic studies of epigenetic modifications | Determine optimal concentration and duration to avoid off-target effects |
| Receptor Antibodies | Anti-ERα, Anti-AR, Anti-PPARγ | Western blot, IHC, ChIP | Validate specificity using knockout controls or siRNA |
| Metabolic Enzymes | CYP450 isoforms, UGT enzymes | Metabolism and bioactivation studies | Use human recombinant enzymes for human health risk assessment |
| Hormone Assays | ELISA/RIA for testosterone, estradiol, TSH | Endpoint analysis in exposed models | Consider cross-reactivity with EDCs in immunoassays |
| Epigenetic Kits | Methylation-specific PCR, ChIP, miRNA isolation | Epigenetic modification analysis | Include bisulfite conversion controls; optimize shearing for ChIP |
The molecular mechanisms of endocrine disruption involve complex, interconnected pathways that span from initial receptor interactions to lasting epigenetic reprogramming. Receptor interference represents the most direct pathway, with EDCs mimicking or blocking endogenous hormones at nuclear receptors critical for development, metabolism, and reproduction [14] [29]. Oxidative stress serves as both a primary mechanism and an amplifier of EDC toxicity, damaging cellular components and activating stress-responsive signaling pathways [33] [31]. Epigenetic modifications provide a plausible explanation for long-term and transgenerational health effects, with EDCs inducing stable changes in gene expression patterns that persist beyond the exposure period [14] [32].
Future research directions should prioritize understanding mixture effects, as real-world exposure invariably involves multiple EDCs simultaneously [14]. Additionally, elucidating the precise mechanisms underlying non-monotonic dose responses will enhance risk assessment accuracy [30]. The development of epigenetic biomarkers for early detection of EDC exposure and affected pathways holds promise for preventive interventions [34] [32]. Integrating multi-omics approaches with advanced bioinformatics will further unravel the complex interplay between EDC exposure, molecular mechanisms, and disease pathogenesis, ultimately informing evidence-based regulatory policies and protective strategies.
The Developmental Origins of Health and Disease (DOHaD) paradigm establishes that environmental exposures during critical developmental windows exert profound influences on long-term health trajectories [35]. Endocrine-disrupting chemicals (EDCs) represent a significant class of environmental stressors capable of crossing the placental barrier and accumulating in fetal tissues, with exposure timing and dosage determining specific physiological outcomes [35]. Emerging evidence indicates that prenatal EDC exposure can reprogram physiological systems through epigenetic mechanisms, potentially creating transgenerational effects that manifest as metabolic disorders, reproductive dysfunction, impaired neurodevelopment, and immune dysregulation later in life [35] [36]. This technical review synthesizes current evidence on life-stage vulnerability to EDCs, detailing specific exposure routes—including dietary, airborne, and dermal pathways—and providing methodological guidance for researchers investigating these critical exposure windows.
Endocrine-disrupting chemicals comprise a diverse group of exogenous substances that interfere with hormone action, synthesis, metabolism, or signaling pathways [1] [37]. These chemicals can mimic, block, or otherwise disrupt the normal function of hormonal systems, particularly during sensitive developmental periods when organizational effects are permanent [35]. The concept of "critical windows of exposure" posits that specific developmental stages—such as prenatal development, early infancy, puberty, and pregnancy—exhibit heightened susceptibility to EDC effects due to rapid cellular differentiation, tissue formation, and metabolic programming [35].
According to the DOHaD framework, environmental stressors encountered during these plastic developmental periods can permanently alter an individual's physiological set points, increasing disease susceptibility across their lifespan [35] [38]. The fetus is particularly vulnerable to EDCs due to immature metabolic capabilities, rapidly developing organ systems, and the absence of fully functional blood-brain and other protective barriers [35]. Numerous EDCs, including bisphenols, phthalates, perfluorinated compounds, and persistent organic pollutants, readily cross the placental barrier, creating a direct exposure pathway to the developing fetus [35] [37].
Dietary Exposure Pathways
Diet represents the most significant exposure route for many EDCs, with chemical migration occurring from food packaging, processing equipment, and environmental contamination [35] [39].
- Food Packaging Materials: Bisphenols (BPA, BPS, BPF) leach from polycarbonate plastics and epoxy resin linings of canned foods and beverages, particularly under heat or acidic conditions [35] [37]. Phthalates migrate from flexible PVC food packaging, especially into fatty foods like meats, dairy products, and oils [35]. Recent analytical studies detected at least one EDC in 144 of 162 screened beverage products, with canned beverages showing significantly higher BPA levels compared to glass or plastic packaging [39].
- Environmental Contaminants: Persistent organic pollutants (POPs), including polychlorinated biphenyls (PCBs) and dioxins, bioaccumulate up the food chain, concentrating in animal fats, fish, and dairy products [35]. Perfluorinated alkyl substances (PFAS) contaminate water sources and migrate from food contact wrappers into food products [37].
- Pesticide Residues: Organophosphate and organochlorine pesticides persist on conventionally grown fruits, vegetables, and grains despite regulatory restrictions in many countries [35].
Non-Dietary Exposure Pathways
- Personal Care Products: Phthalates, parabens, and UV filters in cosmetics, lotions, fragrances, and other personal care items enable dermal absorption and inhalation exposure [1]. The frequency of product use correlates with internal body burden, with certain populations using multiple products daily [1].
- Indoor Air and Dust: Phthalates and brominated flame retardants leach from electronics, furniture, and building materials, partitioning into household dust and enabling inhalation exposure, particularly for children with hand-to-mouth behaviors [37].
- Environmental Justice Considerations: Research following Hurricane Harvey demonstrated that racial/ethnic minorities and communities with lower socioeconomic status experience disproportionately high EDC exposures due to residential proximity to industrial facilities and inadequate infrastructure [40].
Table 1: Primary EDC Classes, Exposure Routes, and Health Concerns
| EDC Class | Major Sources | Primary Exposure Routes | Key Health Concerns |
|---|---|---|---|
| Bisphenols | Food containers, canned linings, receipts, dental sealants | Dietary ingestion, dermal absorption | Neurobehavioral alterations, reproductive dysfunction, metabolic syndrome, PCOS [35] [1] [37] |
| Phthalates | Food packaging, PVC plastics, personal care products, medical tubing | Dietary ingestion, dermal absorption, inhalation | Impaired male reproductive development, asthma, insulin resistance, reduced fertility [35] [37] |
| PFAS | Water-resistant clothing, food wrappers, firefighting foam, cookware | Dietary ingestion, drinking water | Immune disruption, thyroid dysfunction, altered puberty, lipid metabolism disorders [37] [36] |
| Persistent Organic Pollutants | Industrial processes, historical pesticides, burning plastics | Dietary ingestion (animal fats) | Lower birth weight, metabolic dysregulation, cognitive deficits, hormone-sensitive cancers [35] [38] |
| Brominated Flame Retardants | Electronics, furniture foam, building materials, children's toys | Inhalation, dust ingestion | Neurodevelopmental deficits, thyroid disruption, altered pubertal timing [37] |
Health Outcomes Across Developmental Stages
Prenatal and Early Postnatal Development
The prenatal period represents the most vulnerable window for EDC exposure, with effects often manifesting across the lifespan [35]. Key health outcomes associated with prenatal EDC exposure include:
- Metabolic Programming: Prenatal exposure to EDC mixtures associates with lower birth weight z-scores and altered weight trajectories persisting through early childhood [38]. EDCs functioning as environmental obesogens can predispose individuals to weight gain and metabolic disorders through adipocyte differentiation, metabolic set point alteration, and appetite regulation disruption [36]. The SELMA study documented that each unit increase in the EDC mixture index associated with a 0.11 decrease in birth weight z-scores and delayed age at infant peak growth velocity by 0.15 months [38].
- Neurodevelopmental Effects: Bisphenol A exposure associates with increased odds of neurobehavioral problems including anxiety, depression, hyperactivity, and inattention [35] [37]. PBDE flame retardants and certain pesticides associate with impaired cognitive function, psychomotor delays, and attention deficits [37].
- Immune System Programming: Prenatal phthalate exposure associates with approximately doubled risk of childhood wheeze and asthma, with specific metabolites like DEHP increasing asthma odds (OR = 2.03; 95% CI: 1.15–3.57) [35].
- Reproductive System Development: Phthalate exposure during critical genital tubercle formation windows associates with impaired male reproductive development (OR = 1.87; 95% CI: 1.12–3.12 for highest DEHP metabolite quartile) [35]. In females, prenatal EDC exposure may influence ovarian reserve and predispose to polycystic ovary syndrome [1].
Childhood and Adolescence
As children progress through developmental stages, they remain vulnerable to EDC effects due to ongoing brain development, immune maturation, and the onset of pubertal processes:
- Growth Trajectories: The SELMA study demonstrated that prenatal EDC mixture exposure associates with slower infant growth spurt rate (beta = -0.01 on log10 scale) and delayed age at peak growth velocity, with sex-specific effects particularly pronounced in girls [38].
- Puberty and Sexual Maturation: EDCs that interfere with sex steroid signaling, including phthalates, bisphenols, and pesticides, can alter the timing and progression of pubertal development, potentially leading to earlier breast development or delayed pubarche depending on the specific chemical and exposure window [1].
- Obesity Risk: Childhood exposure to certain EDCs, particularly phthalates and bisphenols, consistently associates with increased obesity risk in children, with more heterogeneous associations observed for PFAS and organochlorine pesticides [36].
Transgenerational and Epigenetic Effects
Emerging evidence suggests that EDC exposures during critical developmental windows may produce effects that transcend generations through epigenetic mechanisms [35] [36]. DNA methylation changes, histone modifications, and non-coding RNA expression alterations represent potential mechanisms for the transgenerational inheritance of EDC-induced phenotypes [35]. Experimental models demonstrate that EDCs can promote epigenetic reprogramming of germ cells, enabling the transmission of disease susceptibility to subsequent generations without additional exposure [35].
Methodological Approaches for EDC Research
Exposure Assessment and Mixture Analysis
Advanced exposure assessment methodologies are essential for capturing the complex nature of real-world EDC exposures:
- Biological Sampling: Maternal urine and blood samples during pregnancy provide quantitative exposure biomarkers. The SELMA study analyzed 26 EDCs in prenatal urine and blood samples, measuring specific metabolites of phthalates, phenols, PFAS, PCBs, and pesticides [38].
- Personal Passive Samplers: Silicone wristbands worn for 7-day periods capture complex mixture exposures from multiple routes (dietary, dermal, inhalation), as demonstrated in post-Hurricane Harvey exposure studies [40].
- Mixture Statistical Approaches: Weighted Quantile Sum (WQS) regression enables estimation of mixture effects and identification of "chemicals of concern" within complex exposure profiles [38]. In the SELMA study, WQS regression identified PFOA, Triclosan, HCB, and specific phthalate metabolites as primary drivers of reduced birth weight, while PFOA, BPA, and PCBs dominated effects on growth trajectories [38].
Table 2: Key EDC Mixture Components and Their Contributions to Adverse Outcomes in the SELMA Study [38]
| Health Outcome | Key Contributing EDCs (WQS Weight >3.8%) | Cumulative Contribution | Sex-Specific Differences |
|---|---|---|---|
| Reduced Birth Weight Z-score | PFOA, Triclosan, HCB, 2OHPH, MCiNP, BPS, PFDA, MBP | 74% of WQS index | Minimal sex differences observed |
| Slower Infant Growth Spurt Rate | DPP, PFOA, Triclosan, ΣPCBs, MOiNCH, BPF, PFDA, MEP, 3-PBA | 79% of WQS index | Similar effects in both sexes |
| Delayed Age at Infant Peak Growth Velocity | PFOA, BPA, MOiNCH, MEP, ΣPCBs, DPP, Triclosan, MBzP | 79% of WQS index | Significant in girls (Beta = 0.51 months) but not boys |
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Table 3: Essential Research Materials and Analytical Approaches for EDC Investigation
| Research Tool Category | Specific Examples | Application and Function |
|---|---|---|
| Exposure Assessment | Silicone wristbands (passive samplers), Biomonitoring (urine, blood, amniotic fluid), Food packaging extraction assays | Captures integrated personal chemical exposures; Quantifies internal dose and fetal exposure; Measures leaching potential under various conditions [40] [38] [39] |
| Analytical Chemistry | LC-MS/MS (Liquid Chromatography Tandem Mass Spectrometry), GC-MS (Gas Chromatography-Mass Spectrometry), ICP-MS (Inductively Coupled Plasma Mass Spectrometry) | Identifies and quantifies EDCs and metabolites in biological and environmental samples [38] [39] |
| Statistical Analysis | Weighted Quantile Sum (WQS) regression, Generalized Linear Mixed Models, Effect measure modification analysis | Evaluates mixture effects and identifies driver chemicals; Models growth trajectories over time; Assesses sex-specific vulnerabilities [38] |
| In Vitro Assays | Receptor-mediated reporter gene assays (ERα, AR, TR, PPARγ), Adipogenesis differentiation models (3T3-L1 cells), Epigenetic profiling (bisulfite sequencing) | Screens for endocrine activity; Investigates obesogenic mechanisms; Identifies epigenetic alterations [36] |
| In Vivo Models | Developmental exposure studies (rodents), Transgenerational inheritance models (F0-F3), Xenograft models | Investigates permanent organizational effects; Studies heritable epigenetic changes; Examines carcinogenic potential [35] [36] |
Experimental Protocols for Critical Research Areas
Protocol 1: Assessing Prenatal EDC Mixture Effects on Postnatal Growth Trajectories
This protocol outlines the methodology used in the SELMA study to evaluate associations between prenatal EDC mixtures and children's weight trajectories [38]:
- Study Population Recruitment: Enroll pregnant women during early gestation (weeks 8-13) from prenatal care clinics. Collect comprehensive demographic, medical, and lifestyle data.
- Biological Sample Collection: Obtain maternal urine and blood samples during the first trimester. Process samples within 24 hours and store at -80°C until analysis.
- Chemical Analysis:
- Urinary Biomarkers: Analyze phthalate metabolites, phenol compounds (bisphenols, triclosan), and pesticide metabolites using LC-MS/MS with isotope dilution. Adjust for specific gravity.
- Serum Biomarkers: Quantify PFAS compounds (PFOA, PFOS, PFNA, PFDA) and persistent chemicals (PCBs, OCPs) using HPLC-MS/MS.
- Postnatal Growth Assessment: Measure child weight at birth, 1.5, 3, 4.5, 6, 10, 18, 36, 48, and 60 months. Standardize birth weight for gestational age using WHO references.
- Growth Curve Modeling: Fit the Jenss-Bayley growth model to each child's weight measurements to derive two key parameters:
- Infant growth spurt rate: The exponential rate of growth deceleration during infancy
- Age at infant peak growth velocity (PGV): The age at which growth velocity is maximal
- Statistical Analysis for Mixture Effects:
- Apply WQS regression to evaluate the overall mixture effect, creating a weighted index from all EDCs.
- Use 100 bootstrap samples to estimate chemical weights and identify chemicals of concern.
- Adjust for potential confounders including maternal age, BMI, education, parity, smoking, and child sex.
Protocol 2: Evaluating EDC Transfer From Beverage Packaging Materials
This protocol details methodology for assessing EDC migration from various packaging types into beverages [39]:
- Sample Collection: Acquire 162 non-alcoholic beverages representing different packaging materials (plastic, glass, carton, aluminium, tin cans). Include multiple brands and production batches.
- Extraction and Cleanup:
- Solid-phase extraction (SPE) using Oasis HLB cartridges
- Elution with methanol and concentration under nitrogen stream
- Reconstitution in methanol:water (10:90, v/v) for analysis
- Chemical Analysis:
- Instrumentation: UHPLC-MS/MS system with electrospray ionization
- Analytes: Target 63 EDCs including PFAS, bisphenols, parabens, benzophenones, alkylphenols
- Chromatographic Separation: BEH C18 column (100 mm × 2.1 mm, 1.7 μm) with gradient elution
- Quantification: Use isotope-labeled internal standards for each compound class
- Quality Assurance/Quality Control:
- Include procedural blanks, replicates, and spiked recovery samples
- Use standard addition method for quantification
- Maintain limit of detection (LOD) at 0.1-1.2 ng/L and limit of quantification (LOQ) at 0.3-3.6 ng/L
- Exposure Assessment:
- Calculate estimated daily intake (EDI) = (Chemical concentration × Consumption rate) / Body weight
- Compare EDI with acceptable daily intake (ADI) values, such as EFSA's newly revised BPA guideline of 0.04 ng/kg bw/day
Signaling Pathways and Mechanistic Insights
EDC Mechanisms and Health Outcomes Flow - This diagram illustrates the sequential biological pathways through which EDCs exert their effects, from cellular entry and receptor interactions to epigenetic modifications and ultimate health outcomes across multiple organ systems.
Life-stage vulnerability to EDCs represents a critical determinant of long-term health trajectories, with prenatal and early childhood development constituting particularly sensitive exposure windows. The DOHaD framework provides a mechanistic basis for understanding how EDC exposures during plastic developmental periods reprogram physiological systems, increasing susceptibility to metabolic disorders, reproductive dysfunction, neurodevelopmental deficits, and immune dysregulation [35] [36]. Mixture effects pose particular challenges, as real-world exposures involve complex combinations of chemicals that may interact additively or synergistically [38].
Future research priorities should include:
- Advanced Mixture Methodologies: Developing more sophisticated statistical approaches to decipher complex EDC mixture effects and identify particularly hazardous combinations [38].
- Epigenetic Mechanism Elucidation: Detailed mapping of epigenetic modifications induced by EDC exposures during critical windows and their persistence across generations [35] [36].
- Susceptibility Factor Identification: Understanding genetic, nutritional, and socioeconomic factors that modify vulnerability to EDC effects, particularly in environmental justice communities [40].
- Intervention Strategy Development: Designing evidence-based interventions to reduce EDC exposure during critical windows, particularly for pregnant women and children [35].
The evidence reviewed herein underscores the urgent need for regulatory policies that recognize the unique vulnerability of developing organisms to EDC exposures and implement precautionary approaches to protect those most at risk during critical windows of development.
Advanced Tools for EDC Research: From Biomonitoring to Clinical Data Capture
Validated Survey Instruments for Assessing Exposure-Related Behaviors
Within environmental health research, particularly concerning Endocrine-Disrupting Chemicals (EDCs), accurately quantifying exposure is a critical precursor to understanding health impacts. EDCs are exogenous substances that can interfere with the normal function of the endocrine system, potentially leading to adverse health outcomes such as neurodevelopmental disorders, reproductive issues, metabolic dysfunction, and increased cancer risk [41] [42] [43]. A significant challenge in this field is that exposure to these chemicals occurs through diverse and complex routes, primarily dietary intake, inhalation, and dermal contact [41] [42]. This guide provides an in-depth technical overview of validated survey instruments and methodologies designed to assess these exposure-related behaviors, providing researchers with the tools to generate robust data for exposure assessment and risk characterization.
Validated Survey Instruments and Methodologies
Assessing exposure requires a multi-faceted approach, often combining broad retrospective questionnaires with detailed, real-time data collection. The instruments and methods below represent key tools for capturing the behaviors that influence exposure.
Standardized Recall Instruments
Recall instruments are foundational for collecting data on past behaviors. Their validity hinges on structured design and standardization.
- ACT24 (Activities Completed over Time in 24 Hours): This is a web-based, previous-day recall instrument developed by the National Cancer Institute (NCI). It is specifically designed to estimate daily summaries of physical activity and sedentary behavior, including energy expenditure and time spent in different activity intensities [44]. While focused on activity, its methodology is adaptable for capturing time-activity patterns that influence exposure to environmental contaminants.
- ASA24 (Automated Self-Administered 24-hour Dietary Assessment Tool): Also from the NCI, this is a freely available, web-based tool that enables detailed, self-administered 24-hour dietary recalls. It automatically codes food intake, which is crucial for estimating dietary exposure to EDCs such as bisphenol A (BPA from canned foods), phthalates, and persistent organic pollutants [44] [41]. It features U.S., Canadian, and Australian versions, enhancing its international applicability.
Comprehensive Survey Frameworks for Product and Behavior Analysis
For complex exposure scenarios, more extensive survey frameworks are necessary.
The SUPERB Study (Study of Use of Products and Exposure-Related Behaviors): This longitudinal study was designed to characterize seasonal and long-term changes in exposure-related behaviors. Its multi-platform methodology is a model for comprehensive data collection [45]:
- Telephone Interviews and Internet-Based Surveys: Collected data on food consumption, temporal-spatial activity patterns, and household product use.
- Home-Based Monitoring: Incorporated direct measurement techniques, such as bar scanners and weighing products, to obtain more objective data and reduce recall bias.
- Focus Populations: The study intentionally focused on sensitive populations, including families with young children and older adults, to understand life-stage-specific behaviors [45].
Consumer Product Questionnaire (CPQ) and Recall-Foresight Questionnaire (RFQ): Developed in a feasibility study in Germany, these retrospective questionnaires were designed to retrieve specific, quantifiable data on consumer product use. The parameters collected include use frequency, duration, amounts used, and use conditions for products like cleaning agents, paints, and personal care items [46]. This method was found to provide data directly usable in exposure models like ConsExpo and ECETOC TRA.
Novel and Direct Measurement Approaches
Beyond questionnaires, direct measurement methods can enhance accuracy and provide validation for self-reported data.
- Duplicate Diet and Food Sampling: In a pilot study on microplastic exposure, researchers used duplicate meal methods and questionnaires to document dietary and water intake behaviors. The food and water consumed were physically sampled and later analyzed in the lab, providing a direct link between behavior and environmental concentration [47].
- Protocols and Video Documentation: The German feasibility study compared questionnaires with "task" methods, where participants filled out written protocols or made video recordings during product use. This approach was found to yield highly detailed and valid information on actual use practices, which can differ significantly from recalled behavior [46].
Table 1: Summary of Key Validated Survey Instruments and Their Applications
| Instrument Name | Data Collection Method | Primary Exposure Behaviors Assessed | Key Advantages |
|---|---|---|---|
| ASA24 [44] | Automated 24-hour dietary recall | Dietary intake | Freely available, automated coding, multiple country versions |
| ACT24 [44] | Previous-day activity recall | Physical activity and sedentary behavior | Estimates energy expenditure and time allocation |
| SUPERB Framework [45] | Multi-platform (phone, online, home monitoring) | Food consumption, product use, activity patterns | Longitudinal design captures seasonal variation |
| CPQ/RFQ [46] | Retrospective questionnaire | Consumer product use frequency, duration, and amount | Data is directly usable in exposure models (e.g., ConsExpo) |
| Duplicate Diet Method [47] | Food sampling & questionnaires | Dietary intake | Directly links consumption to contaminant concentrations |
Experimental Protocols for Exposure Assessment
Implementing a rigorous exposure assessment study requires carefully designed protocols. The following are detailed methodologies drawn from cited research.
Protocol for a Longitudinal Behavior Survey (SUPERB)
The SUPERB study provides a robust protocol for capturing a wide range of exposure-related behaviors over time [45].
- Study Population and Sampling: Employ probability-based sampling to ensure representativeness. SUPERB recruited two distinct cohorts: a) families with young children from birth certificate records, and b) older adults from tax assessor records. Oversampling of underrepresented subgroups (e.g., based on maternal education) is critical to ensure a diverse sample [45].
- Tiered Data Collection:
- Tier 1 - Questionnaires: Administer core questionnaires (e.g., CPQ) via telephone or online to collect baseline data on product use and behaviors over the past 12 months.
- Tier 2 - Follow-up and Detailed Reporting: Several weeks later, administer a second questionnaire (e.g., RFQ) focusing on the past 4 weeks to allow for comparison of recall periods. From this pool, recruit participants for detailed "tasks."
- Tier 3 - Direct Documentation: Provide participants who intend to use a target product with a written protocol to complete during use or an action camera to video record the use event. This captures precise data on amounts, duration, and conditions [46].
- Data Management and Analysis: Data should be handled to calculate descriptors like means, medians, and percentiles (e.g., 75th, 95th) essential for exposure modeling. Analysis should account for intra-individual variability over time [45].
Protocol for Integrated External and Internal Exposure Assessment
This protocol, adapted from a microplastics pilot study, is highly applicable for assessing EDCs where both environmental and biomonitoring data are needed [47].
- Participant Recruitment and Questionnaire: Recruit a defined participant group (e.g., 26 college students). Use a questionnaire to document their typical dietary intake, water consumption, and activity locations.
- Environmental Sampling (External Exposure):
- Dietary Intake: Apply the duplicate diet method. Collect and weigh duplicates of all food and beverages consumed by participants over a 24-hour period.
- Water Intake: Collect samples of all drinking water consumed.
- Inhalation Exposure: Use air samplers to collect indoor and outdoor air particulate matter from the participants' primary activity regions (e.g., home, workplace).
- Biological Sampling (Internal Exposure): Collect fasting blood serum, 24-hour urine, and fecal samples from participants following the environmental sampling period.
- Laboratory Analysis: Analyze all environmental and biological samples using appropriate chemical analytical methods, such as Pyrolysis Gas Chromatography/Mass Spectrometry (Py-GCMS) for polymers or LC-MS/MS for specific EDCs, to identify and quantify the contaminants of interest [47].
- Exposure Estimation: Calculate the external exposure dose via dietary intake, water intake, and inhalation (e.g., in μg/kg bw/d). Correlate these values with the internal exposure levels measured in the biological matrices.
The Scientist's Toolkit: Research Reagent Solutions
Successful exposure assessment relies on a suite of methodological "reagents" – both physical and digital. The following table details key resources for building and executing these studies.
Table 2: Essential Research Tools for Exposure Behavior Assessment
| Tool / Resource | Function / Description | Application in Exposure Research |
|---|---|---|
| ASA24 & ACT24 (NCI) [44] | Freely available, automated 24-hour recall systems for diet and activity. | Standardized assessment of primary dietary and time-activity exposure pathways. |
| ConsExpo & ECETOC TRA [46] | Consumer exposure modelling software tools. | Use output from surveys (frequency, amount) to quantify chemical exposure and risk. |
| Pyrolysis GC/MS (Py-GC/MS) [47] | Analytical chemistry technique for identifying and quantifying polymers and complex organics. | Used to measure microplastics and other complex contaminants in environmental and biological samples. |
| Duplicate Diet Method [47] | Collection of duplicate portions of all food and drink consumed. | Provides the most accurate link between dietary consumption and contaminant intake. |
| Stratum Corneum (SC) Permeability Assay [48] | Ex vivo measurement of skin barrier integrity and compound penetration. | Evaluates dermal absorption potential of EDCs and effects of product formulations on skin barrier. |
| Structured Questionnaires (CPQ/RFQ) [46] | Retrospective surveys designed for quantifiable data on product use. | Captures behavioral parameters (frequency, amount) for input into exposure models. |
Data Presentation and Analysis
Translating raw survey and analytical data into actionable information requires systematic processing and clear presentation.
- Data Processing for Exposure Models: Survey data on frequency and amount of product use must be processed to generate central tendencies and distribution metrics. Exposure assessments require mean, median, and high-percentile (e.g., 75th, 95th) values to describe both average and extreme exposure scenarios [46].
- Calculating Exposure Doses: For chemicals, the Systemic Exposure Dose (SED) is calculated using data on product use amount, frequency, and chemical concentration. This SED is then compared to a toxicity reference value (e.g., NOAEL - No Observable Adverse Effect Level) to derive a Margin of Safety (MoS) [49].
Table 3: Quantitative Exposure Data from a Microplastics Pilot Study [47]
| Exposure Pathway / Matrix | Analytes Detected | Median / Mean Concentration | Calculated Exposure Dose |
|---|---|---|---|
| Dietary Intake | Various Microplastics | 2.50 - 91.30 μg/g (in food) | 346.65 μg/kg bw/day |
| Water Intake | Various Microplastics | 0.02 - 18.41 μg/g (in water) | 41.17 μg/kg bw/day |
| Inhalation | PS, PE, PP, PVC, PET (in air) | Not Specified | 59.57 μg/kg bw/day |
| Total External Exposure | - | - | 460.20 μg/kg bw/day |
| Serum (Internal) | Various Microplastics | 20.81 μg/g (median) | - |
| Urine (Internal) | PS, PE, PP, PVC, PET, PA66, PMMA | 5.06 μg/g (median) | - |
Biomonitoring serves as a critical tool in environmental health, enabling the direct measurement of chemical exposures and their metabolites within human biological matrices. For researchers investigating exposure to endocrine-disrupting chemicals (EDCs), selecting appropriate biomonitoring techniques is paramount for accurately assessing body burden, understanding exposure routes, and elucidating potential health effects. EDCs—substances that interfere with hormonal signaling—enter the body primarily through food, air, and skin absorption, posing risks to reproductive, metabolic, and developmental health [8] [18] [36]. This technical guide provides an in-depth analysis of established and emerging biomonitoring methodologies, focusing on blood, urine, and non-invasive alternatives within the context of EDC research.
The fundamental principle of biomonitoring relies on measuring contaminants or their biomarkers in biological specimens to evaluate internal dose and potential health risks. Blood and urine have traditionally dominated human biomonitoring studies; however, non-invasive matrices like saliva, hair, and nails are gaining prominence for their ability to improve participant compliance, especially in vulnerable populations and large-scale studies [50]. Advances in analytical technologies now permit sensitive measurement of EDCs and trace elements in these alternative matrices, expanding the toolkit available to researchers and clinicians.
Established Biomonitoring Matrices: Blood and Urine
Blood as a Gold Standard Matrix
Blood represents the ideal matrix for many biomonitoring applications due to its dynamic equilibrium with tissues and organs throughout the body. It provides a comprehensive picture of systemic exposure, reflecting recent and cumulative body burden for many persistent chemicals [50]. Blood is particularly valuable for assessing lipophilic EDCs—such as polychlorinated biphenyls (PCBs), organochlorine pesticides, and polybrominated diphenyl ethers (PBDEs)—that accumulate in lipid-rich tissues and circulate in lipoproteins [50]. Measurements in blood can include parent compounds, metabolites, and protein adducts, offering insights into both exposure and metabolic processing.
However, blood collection presents significant limitations: it is invasive, requires trained phlebotomists, poses infection risks if not properly handled, and may encounter participant reluctance in volunteer studies [50]. These factors can limit sample size and population representativeness, particularly in longitudinal studies requiring repeated measurements.
Urine as a Practical Alternative
Urine serves as the most widely used non-invasive matrix for monitoring exposure to non-persistent chemicals with short biological half-lives. It is particularly suitable for measuring water-soluble metabolites of phthalates, bisphenols, parabens, and pesticides, which are rapidly excreted via renal clearance [50] [18]. Urine collection is simple, low-risk, and acceptable to most participants, enabling large-scale population studies and repeated sampling to account for temporal exposure variations.
A key consideration in urinary biomonitoring is the adjustment for dilution using specific gravity or creatinine correction [51]. This normalization is essential for comparing results across individuals and sampling times. While urine excellently captures recent exposure (hours to days), it generally reflects neither body burden nor historical exposure to persistent organic pollutants [50].
Blood-Urine Correlation for Trace Elements
Understanding the relationship between concentrations in blood and urine informs matrix selection, particularly for trace elements. A 2025 pilot biomonitoring study with school-aged children evaluated correlations for 17 elements, finding significant associations for only three, as summarized in Table 1 [51].
Table 1: Correlation Between Blood and Urine Levels of Select Trace Elements in Children
| Element | Spearman's rho (ρ) | 95% Confidence Interval | Implication for Biomonitoring |
|---|---|---|---|
| Lead (Pb) | 0.43 | 0.24–0.61 | Moderate correlation; urine may serve as alternative for exposure assessment |
| Arsenic (As) | 0.23 | 0.01–0.44 | Weak correlation; matrix selection depends on research question |
| Strontium (Sr) | 0.22 | 0.03–0.40 | Weak correlation; interpretation requires caution |
| Other elements (Al, Ba, Cs, etc.) | Not significant | - | Blood and urine measure different exposure windows/compartments |
These findings underscore that urine cannot universally substitute for blood in biomonitoring. For lead, the moderate correlation suggests urine could complement blood measurements in large-scale studies where participant compliance is paramount [51]. For other elements, the lack of significant correlation indicates different pharmacokinetics, with blood often reflecting different exposure timelines or internal compartments than urine.
Emerging Non-Invasive Matrices and Technologies
Alternative Non-Invasive Matrices
Several biological matrices offer non-invasive alternatives for specific research applications, each with distinct advantages and limitations for EDC biomonitoring, as detailed in Table 2.
Table 2: Non-Invasive Matrices for Biomonitoring of EDCs and Trace Elements
| Matrix | Analytes | Advantages | Limitations | Research Applications |
|---|---|---|---|---|
| Hair | Heavy metals (Pb, Hg, Cd), certain POPs, drugs [50] | Provides long-term exposure history (weeks to months); simple storage and transport; minimal infection risk [50] | Potential for external contamination; not suitable for volatile compounds; limited reference values [50] | Chronic exposure assessment; geographical studies; historical exposure reconstruction |
| Saliva | Steroid hormones, therapeutic drugs, alcohol, nicotine [50] | Simple collection; suitable for frequent sampling; measures bioavailable fraction [50] | Low analyte concentrations; dilution variability; limited data for EDCs [50] | Real-time monitoring; pediatric studies; occupational settings |
| Nails | Heavy metals (As, Se, Hg), trace elements [50] | Very stable matrix; reflects exposure over months; easy storage [50] | Slow growth rate; external contamination concerns; requires rigorous cleaning [50] | Chronic exposure assessment; epidemiological studies |
| Breast Milk | Persistent lipophilic compounds (PCBs, PBDEs, DDT, dioxins) [50] | Provides infant exposure data; reflects maternal body burden; non-invasive for infant [50] | Only available from lactating women; lipid content varies; ethical considerations [50] | Infant exposure assessment; maternal body burden estimation |
Advanced Biomonitoring Technologies
Recent technological innovations are expanding non-invasive biomonitoring capabilities, particularly for women's health. Wearable sensors now enable continuous, real-time monitoring of physiological parameters relevant to EDC exposure and effects. These include:
- Intravaginal loggers for precise basal body temperature tracking, providing superior accuracy for ovulation detection compared to wrist-worn devices [52]
- Smart textiles with integrated sensors to monitor uterine contractions, maternal heart rate, and respiration during pregnancy [52]
- Electrochemical biosensors in development for interstitial fluid analysis, potentially enabling non-invasive measurement of EDCs or their biomarkers [52]
These technologies facilitate long-term biomonitoring in naturalistic settings, capturing dynamic responses to environmental exposures and enabling personalized exposure assessments.
EDC-Specific Biomonitoring Considerations
EDC Exposure Routes and Matrix Selection
Understanding EDC exposure routes—food, air, and skin absorption—is essential for designing effective biomonitoring strategies. Each route may involve different congeners, metabolites, and temporal exposure patterns, necessitating careful matrix selection [13] [18].
Food-borne exposures typically involve lipophilic persistent compounds (e.g., PCBs, dioxins) best measured in blood or breast milk, or non-persistent compounds (e.g., BPA, phthalates) optimally measured in urine [50] [18]. Inhalation exposures to volatile compounds or particulate-bound EDCs may be assessed through blood or urine, while dermal exposures to compounds like phthalates in personal care products are effectively tracked in urine samples [13].
The development of validated exposure surveys, such as the 19-item reproductive health behavior questionnaire for EDC exposure assessment through food, respiratory, and dermal routes, provides complementary tools for estimating exposure sources when biomonitoring is not feasible [13].
EDC Mechanisms and Biomarker Selection
EDCs disrupt hormonal homeostasis through multiple mechanisms, informing biomarker selection in biomonitoring studies:
- Receptor-mediated effects: BPA and phthalates act as estrogen receptor agonists/antagonists or androgen receptor antagonists [53]
- HPG axis disruption: EDCs alter GnRH secretion, affecting LH and FSH production with downstream effects on steroidogenesis [53]
- Epigenetic modifications: Early-life EDC exposure induces heritable changes in gene expression through DNA methylation and histone modification [36] [53]
- Oxidative stress: Many EDCs generate reactive oxygen species, impairing sperm function and testicular physiology [53]
These mechanisms support measuring not only parent EDCs and metabolites but also downstream biomarkers like hormone levels, DNA methylation patterns, and oxidative stress markers for comprehensive risk assessment.
Experimental Protocols and Methodologies
Standardized Biomonitoring Protocol for Trace Elements
The following protocol, adapted from a 2025 pediatric biomonitoring study, details standardized procedures for collecting and analyzing blood and urine samples for trace element assessment [51]:
Sample Collection:
- Urine: Collect morning spot-urine samples via spontaneous micturition in sterile 150mL containers. Record specific gravity for dilution adjustment. Store immediately at -4°C until analysis.
- Blood: Collect 4mL venous blood using peripheral venipuncture into K₂EDTA Vacutainer tubes (lavender cap). Store at 4°C until processing.
Sample Preparation:
- Urine Processing: Thaw samples and aliquot 200μL. Dilute 1:10 with 65% nitric acid (Merck) and ultrapure water to achieve final acid concentration of 0.16% (v/v).
- Blood Processing: Digest samples using acid digestion with microwave irradiation (Milestone Ethos One equipment).
Element Quantification:
- Analyze prepared samples using ICP-MS NexION 300D (Perkin Elmer).
- Calibrate using multi-element calibration standards 2, 3, 4, and 5 (P/N N9300232, N9300233, N9300234, N9300235).
- Run samples in duplicate with calibration curve consisting of blank and six concentrations (0.5, 1, 5, 10, 25, 50, and 100 ng/mL).
- Follow validated method LISTO-MET-PRO-TEC-016 from Metal Laboratory of CINVESTAV, Mexico.
Quality Control:
- Include procedural blanks, duplicate samples, and certified reference materials in each batch.
- Monitor instrument performance with internal standards.
- Apply specific gravity correction for urine samples to account for dilution variations.
Research Reagent Solutions and Essential Materials
Table 3: Essential Research Reagents and Materials for Biomonitoring Studies
| Item | Specification/Function | Application Notes |
|---|---|---|
| Sterile Collection Containers | 150mL capacity for urine samples | Pre-screened for trace element contamination [51] |
| K₂EDTA Vacutainer Tubes | Lavender cap, 4mL volume | Prevents coagulation for blood metal analysis [51] |
| Nitric Acid | 65% purity (Merck, Darmstadt) | Sample digestion and preservation; ultrapure grade required [51] |
| ICP-MS Instrument | Perkin Elmer NexION 300D | Multi-element quantification at ng/mL levels [51] |
| Multi-element Calibration Standards | P/N N9300232, N9300233, N9300234, N9300235 | Six-point calibration (0.5-100 ng/mL) for 17 elements [51] |
| Microwave Digestion System | Milestone Ethos One | Complete digestion of blood samples prior to ICP-MS [51] |
| Specific Gravity Refractometer | Urine density measurement | Correction for dilution in spot urine samples [51] |
Biomonitoring techniques utilizing blood, urine, and non-invasive matrices provide complementary approaches for assessing EDC exposure through food, air, and skin absorption routes. Selection of appropriate matrices depends on research objectives, chemical properties of target analytes, exposure timing, and practical considerations regarding participant burden and compliance.
Blood remains the gold standard for assessing body burden of persistent lipophilic compounds, while urine excels for capturing recent exposure to non-persistent EDCs. Emerging non-invasive matrices like hair, saliva, and nails offer specialized applications for specific exposure assessment scenarios. Advanced technologies including wearable sensors and point-of-care devices represent the future of personalized biomonitoring, enabling real-time exposure assessment and intervention.
For researchers investigating EDC exposure routes, a multi-matrix approach often provides the most comprehensive exposure assessment, particularly when combined with validated exposure questionnaires. As biomonitoring technologies continue to evolve, they will enhance our ability to quantify EDC exposures, elucidate biological mechanisms, and inform evidence-based public health interventions to reduce environmental health risks.
Leveraging Electronic Data Capture (EDC) Systems for Clinical Trial Data
Electronic Data Capture (EDC) systems are web-based software platforms that have revolutionized clinical trials by replacing paper-based case report forms (CRFs) with electronic data collection, enabling real-time data access, improved quality, and regulatory compliance [54] [55]. For researchers investigating exposure routes—such as food, air, and skin absorption—EDC systems provide a robust framework for managing complex, multi-source data, ensuring the integrity and traceability required for high-quality environmental and public health research.
Core Functions and System Architecture of an EDC
At its core, an EDC system is designed to collect, manage, and clean clinical trial data electronically [55]. Its primary functions include:
- Data Entry: Provides a user-friendly interface for researchers to input participant data directly into electronic Case Report Forms (eCRFs), often allowing remote access to eliminate transcription errors [55].
- Data Validation: Performs automated, real-time checks to ensure data falls within expected parameters, flagging inconsistencies or missing values immediately upon entry [55] [56].
- Data Storage and Security: Maintains all data in a centralized, secure database, often cloud-based, with features like audit trails, data encryption, and access controls to comply with regulations like 21 CFR Part 11 and HIPAA [55] [57].
The system architecture supporting these functions integrates multiple components, from user management to data processing, as shown in the workflow below.
Quantitative Comparison of Leading EDC Systems
Selecting an appropriate EDC system requires evaluating key features against study needs. The table below summarizes enterprise-grade platforms suitable for large-scale studies, including complex exposure research.
| EDC System | Key Strengths | Ideal Use Case | Compliance |
|---|---|---|---|
| Medidata Rave EDC | Global scale, AI-powered forecasting, integrates with eCOA, RTSM, eTMF [54]. | Large global trials (e.g., oncology, CNS) [54]. | 21 CFR Part 11, ICH-GCP [54]. |
| Oracle Clinical One EDC | Unifies randomization, supplies, EDC; real-time access; supports mid-study updates [54]. | Trials needing a unified platform for data and supplies [54]. | Global data privacy laws, 21 CFR Part 11 [54]. |
| Veeva Vault EDC | Cloud-native, rapid study builds, drag-and-drop CRF, connects to CTMS & eTMF [54]. | Sponsors seeking an end-to-end unified platform [54]. | 21 CFR Part 11, ICH-GCP [54]. |
| Castor EDC | Rapid startup, prebuilt templates, eConsent, patient-reported outcomes [54]. | Academic institutions, decentralized trials [54]. | 21 CFR Part 11, GDPR [54]. |
| IBM Clinical Development | AI-powered discrepancy detection, remote SDV, mobile eConsent, built for scale [54]. | CROs managing hundreds of sites [54]. | 21 CFR Part 11, HIPAA [54]. |
For research with budget constraints or in academic settings, platforms like REDCap (free for academic research) and OpenClinica Community Edition (open-source) offer robust EDC functionality without high licensing costs [54].
Experimental Protocol for Data Capture in Exposure Route Studies
Implementing an EDC system for a study on skin absorption of a compound involves a structured, multi-stage protocol.
Study Configuration and eCRF Design
- Create Study Project: In the EDC system (e.g., REDCap), create a new longitudinal project to support multiple participant visits over time [56].
- Design Electronic Case Report Forms (eCRFs): Use the EDC's online designer to create eCRFs with fields for demographics, exposure history, and clinical measurements. Employ branching logic to show/hide fields based on previous answers (e.g., skip questions on inhalation if exposure route is dermal) [56].
- Implement Data Validation: Configure field validation rules (e.g., date formats, numerical ranges for lab values) to enforce data quality at the point of entry [56].
Data Collection and Management Workflow
The following diagram illustrates the sequential flow of data from collection through to analysis, highlighting automated quality checks.
Data Integration, Monitoring, and Lock
- Integrate External Data: Use the EDC's API interfaces or custom scripts to automatically import laboratory results (e.g., blood concentration levels) from Lab Information Management Systems (LIMS) or electronic health records (EHRs), reducing manual entry [58].
- Monitor and Clean Data: Investigators and data managers use the EDC's reporting tools to monitor data completeness and resolve any queries that arise [55].
- Lock Database: Once all data is cleaned and queries resolved, the database is locked to finalize the dataset for analysis [59].
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful execution of exposure studies relies on both the digital EDC platform and physical research materials. The following table details key reagents and their functions.
| Item | Function in Exposure Route Research |
|---|---|
| Biomarker Assay Kits | Detect and quantify specific compounds or their metabolites in biological samples (e.g., blood, urine) to confirm and measure exposure [56]. |
| Dermal Patches | Standardized application of a compound to the skin for controlled absorption studies [54]. |
| Environmental Sampling Pumps | Collect airborne particulates or vapors for subsequent analysis of inhalation exposure levels [58]. |
| Standardized Food/Water Matrices | Used in dietary exposure studies to ensure consistent and measurable compound administration [56]. |
| Data Integration Middleware | Custom scripts or software that enable EDC systems to pull data from external sources like electronic health records (EHR) or lab systems, automating data flow [58]. |
Data Integration and Management for Multi-Source Studies
Exposure research often requires synthesizing data from disparate sources. A centralized integration approach is critical.
- EMR/EDC Integration: Automatically pull relevant patient history and baseline health data from Electronic Medical Records (EMRs) into the EDC system, reducing double data entry and transcription errors [58].
- Wearable and Sensor Data Integration: Ingest data from wearable devices that monitor physiological parameters (e.g., heart rate, activity) to provide continuous, objective measures of participant status and potential reactions [58].
- Laboratory Data Integration: Automatically import laboratory results from LIMS, ensuring timely and accurate availability of biomarker or toxicology data for analysis [58].
The diagram below illustrates how an EDC system acts as the central hub for these diverse data streams in an exposure study.
EDC systems are indispensable for modern clinical research, providing the structure and rigor necessary for generating reliable evidence. For scientists investigating exposure routes, these platforms offer a powerful means to manage complex data workflows, integrate diverse data types, and maintain unwavering data integrity from collection through analysis. By leveraging the automated validation, centralized data management, and integration capabilities of EDC systems, researchers can accelerate timelines and enhance the quality of their findings, ultimately contributing to a deeper understanding of environmental health impacts.
The field of direct data capture is undergoing a revolutionary transformation, driven by advancements in wearable sensor technology and sophisticated data analytics. For researchers investigating exposure routes of endocrine-disrupting chemicals (EDCs)—through food, air, and skin absorption—these technologies offer unprecedented capabilities for real-time, continuous monitoring of both environmental exposures and their biological effects. EDCs are synthetic chemicals that interfere with the body's hormonal system, entering through various exposure routes and posing threats to reproductive health, including infertility and cancer [42] [60]. Modern wearable devices have evolved far beyond basic fitness trackers into sophisticated diagnostic platforms that provide critical insights into physiological changes and chemical exposure biomarkers, enabling a more precise understanding of the relationship between EDC exposure and health outcomes [61] [62].
The shift to these technologies represents a move from episodic, reactive healthcare to personalized, preventive medicine [61]. For researchers, this means transitioning from traditional methods like infrequent blood or urine analyses to continuous, real-time biomarker monitoring that captures dynamic changes in both exposure and physiological response [63]. This technical guide explores the core technologies, experimental methodologies, and data integration frameworks shaping this evolving landscape for research professionals and drug development specialists working specifically in the realm of EDC exposure and its health impacts.
Wearable Sensor Technology for Exposure and Effect Monitoring
Core Components and Sensing Modalities
Next-generation wearable sensors are engineered with sophisticated components that enable precise, continuous monitoring of physiological signals and biochemical markers. The architecture of these devices typically integrates several core subsystems:
Biosensors: Modern wearables incorporate miniaturized biosensors capable of tracking a wide array of physiological parameters including heart rate, oxygen saturation, body temperature, respiration, and galvanic skin response [61]. For EDC research, emerging sensors can measure drug concentrations and specific biomarkers in biofluids like sweat, offering potential for non-invasive therapeutic drug monitoring and exposure assessment [63]. These sensors operate through various mechanisms including electrochemical detection (e.g., enzyme-based chronoamperometry, cyclic voltammetry) and optical sensing [63].
Microprocessors and AI Chips: At the core of these devices are powerful processors that analyze collected data in real-time. Artificial intelligence (AI) chips enable pattern detection, anomaly recognition, and predictive insights by processing complex biomarker data streams, sometimes without needing external apps [61]. This capability is crucial for identifying subtle physiological changes resulting from EDC exposure that might follow non-linear dose-response relationships [60].
Connectivity Modules: Wireless technologies including Bluetooth, 5G, and Wi-Fi ensure seamless data transmission from wearable devices to mobile applications, cloud storage, and research platforms [61]. This enables real-time communication between study participants and research teams, facilitating immediate data access and analysis.
Power Systems: Long-life batteries with power-saving chips are essential for continuous monitoring. Some advanced devices now incorporate solar-assisted or motion-based energy harvesting, allowing extended operation between charges [61].
Advanced Materials: Wearables now utilize skin-safe, flexible polymers, breathable smart fabrics, and hypoallergenic materials that enable 24/7 wear without irritation, supporting longitudinal study designs [61]. The development of "smart fabrics" represents a particularly promising advancement, with clothing embedded with biosensors that can track electrophysiological signals, movement, temperature, and hydration without the bulk of traditional devices [61].
Wearable Formats and Research Applications
Wearable sensors for research deployment come in various form factors, each with distinct advantages for exposure science and health effect monitoring:
Table 1: Wearable Device Categories for Exposure and Health Monitoring Research
| Device Type | Primary Monitoring Capabilities | Research Applications |
|---|---|---|
| Smartwatches/Fitness Bands | ECG, heart rate variability, oxygen saturation, physical activity, sleep patterns | Monitoring physiological stress responses to EDC exposure; tracking activity-rest patterns in relation to exposure biomarkers |
| Continuous Glucose Monitors | Real-time glucose levels | Metabolic disruption studies; correlation with EDC exposure timing |
| Smart Patches | Medication levels, vital signs, sweat biomarkers | Therapeutic drug monitoring; pharmacokinetic studies of EDC metabolism; dermal absorption studies |
| AI Hearing Aids | Sound exposure, physiological parameters | Noise exposure studies; stress response monitoring |
| Smart Clothing | Posture, breathing patterns, muscle activity, electrophysiological signals | Unobtrusive continuous monitoring in natural environments; occupational exposure studies |
For EDC research specifically, wearable sensors present a transformative opportunity to move beyond traditional exposure assessment methods. While current approaches often rely on infrequent blood or urine analysis [60], wearable sensors enable continuous, real-time measurement of dynamic changes in drug levels or stress biomarkers in biofluids like sweat [63]. This is particularly valuable given that the effects of EDC exposure vary by life stage and gender, and current exposure limits may not fully protect vulnerable populations like couples trying to conceive [60].
Experimental Protocols for Wearable Sensor Deployment
Study Design and Sensor Implementation Framework
Implementing wearable sensors in exposure science research requires meticulous study design and validation protocols. The following workflow outlines a systematic approach for deploying wearable technologies in studies investigating EDC exposure routes and health effects:
Diagram 1: Wearable Study Implementation Workflow
The experimental workflow begins with precise definition of study objectives, which dictates sensor selection. For EDC research, this might involve selecting sensors capable of detecting physiological stress responses or specific biomarkers of effect. The validation phase is critical and must encompass both technical performance verification and clinical validation against established reference methods [60]. For example, in monitoring levodopa concentrations for Parkinson's patients, researchers have validated sweat-based measurements against high-performance liquid chromatography analysis of blood samples, demonstrating a correlation of 0.678 between the two matrices [63].
Participant training ensures proper device usage and adherence to protocols, which is especially important in longitudinal exposure studies. The baseline data collection phase establishes individual reference points against which changes can be measured during the continuous monitoring phase. Throughout active monitoring, compliance tracking and data quality verification are essential to ensure dataset integrity [60]. The final data integration and analysis phase employs specialized statistical approaches to handle high-frequency longitudinal data and identify exposure-response relationships.
Biomarker Detection Methodologies
Specific experimental protocols vary based on the target biomarkers and analytical approaches. The following diagram illustrates a generalized methodology for continuous biomarker monitoring using wearable sensors:
Diagram 2: Biomarker Detection Methodology
For biochemical monitoring (e.g., drugs, hormones, stress biomarkers), electrochemical detection methods are commonly employed. For instance, researchers have developed wearable enzyme-based electrochemical biosensors for real-time detection of levodopa in sweat. These sensors utilize a screen-printed carbon paste substrate with immobilized tyrosinase on the surface to create a specific working electrode. When sweat is collected via a hydrogel covering, the electrode detects levodopa through oxidation by tyrosinase, generating a measurable electrochemical signal [63]. Such sensors have demonstrated detection limits as low as 300 nM for target analytes and shown pharmacokinetic profiles comparable to blood sampling [63].
For EDC research specifically, wearable sensors could be deployed to monitor physiological stress responses or effect biomarkers while simultaneously collecting exposure data through complementary methods. This approach addresses the challenge that EDCs enter the body through multiple routes (food, air, skin) and their effects vary by timing and duration of exposure [42] [60]. The continuous data streams enable researchers to capture transient exposure events and corresponding physiological responses that would be missed with traditional biomonitoring approaches.
Data Management, Validation, and Integration
Electronic Data Capture (EDC) Systems for Wearable Data
The high-volume, high-velocity data generated by wearable sensors requires robust electronic data capture (EDC) systems specifically designed for clinical research. Modern EDC systems provide the infrastructure to collect, manage, and store clinical trial data electronically, replacing traditional paper-based methods [55]. These systems typically consist of three core components:
Data Entry Interfaces: User-friendly portals that allow researchers to input supplementary data and enable integration with wearable device APIs for automated data ingestion from multiple sensor sources [55].
Data Validation Engines: Automated systems that perform real-time validation checks on incoming data, identifying inconsistencies, outliers, or missing values immediately to ensure data quality at the point of entry [55]. These systems employ built-in range checks and logical validation to flag physiologically implausible values.
Centralized Secure Databases: Cloud-based repositories that aggregate wearable data with other clinical datasets while maintaining strict security protocols, encryption, and access controls compliant with regulations like 21 CFR Part 11 and GDPR [55] [54].
Leading EDC platforms such as Medidata Rave, Oracle Clinical One, and Veeva Vault EDC offer specialized capabilities for handling wearable device data, including support for high-frequency data streams, integration with analytics platforms, and tools for remote monitoring of data quality [54].
System Validation and Regulatory Compliance
For research intended for regulatory submission, validation of the entire data capture system—including wearable sensors and EDC platforms—is essential. Validation ensures that these systems function as intended and meet regulatory requirements for data integrity and security [64]. The validation process typically involves:
User Acceptance Testing (UAT): Researchers test the system with realistic data scenarios to verify that all components work together as expected, including data flow from wearables through the EDC system to analytical outputs [64].
Risk-Based Validation Approaches: Rather than validating every feature after each update, researchers can adopt risk-based approaches that focus validation efforts on system components that impact critical data integrity or patient safety [65]. This involves reviewing system updates, testing features that affect core research workflows, and thoroughly documenting decisions.
Regulatory bodies like the FDA encourage risk-based approaches to data management, allowing research teams to focus resources on the most critical data points rather than attempting comprehensive review of all data points [66]. For wearable sensor data, this might mean prioritizing validation of algorithms that detect clinically significant events or biomarkers rather than validating every aspect of raw data processing.
Table 2: Quantitative Performance Metrics for Wearable Sensors in Research Settings
| Performance Parameter | Typical Range | Validation Methodology | Considerations for EDC Research |
|---|---|---|---|
| Analytical Accuracy | 90-98% vs. gold standard | Method comparison studies with Bland-Altman analysis | Critical for establishing exposure-response relationships |
| Precision (Coefficient of Variation) | 3-8% for most analytes | Repeated measurements of quality control materials | Determines ability to detect subtle physiological changes |
| Limit of Detection | Compound-dependent (e.g., 300 nM for levodopa) | Signal-to-noise ratio determination | Must be sufficient for detecting biologically relevant EDC effects |
| Sensor Response Time | Seconds to minutes for continuous monitoring | Measurement of time to stable signal | Important for capturing acute exposure events |
| Inter-Sensor Reproducibility | 5-12% variance between units | Testing multiple devices simultaneously | Essential for multi-site studies |
| Operational Battery Life | 24-72 hours continuous use | Continuous operation testing | Impacts compliance in longitudinal exposure studies |
Research Implementation Toolkit
Essential Research Reagents and Materials
Implementing wearable sensor research requires specific materials and reagents tailored to the exposure and outcome markers of interest. The following table details key components for a comprehensive research toolkit:
Table 3: Research Reagent Solutions for Wearable Sensor Studies
| Item Category | Specific Examples | Research Function | Technical Considerations |
|---|---|---|---|
| Biosensing Elements | Tyrosinase, glucose oxidase, lactate oxidase, antibody fragments | Biomarker recognition and signal generation | Select based on specificity for target EDC biomarkers or effect markers |
| Sensor Substrates | Screen-printed carbon electrodes, gold nanoparticles, graphene inks | Transduction platform for biochemical signals | Impact sensitivity, reproducibility, and manufacturing scalability |
| Interface Materials | Hydrogels, microneedle arrays, cellulose membranes | Biofluid collection and sample conditioning | Influence sampling rate and biocompatibility for long-term wear |
| Calibration Solutions | Buffer solutions with known analyte concentrations | Sensor calibration and performance verification | Essential for maintaining measurement accuracy across study duration |
| Reference Materials | Certified reference materials for target analytes | Method validation and quality control | Crucial for establishing measurement traceability |
| Data Processing Tools | Custom algorithms for signal filtering, feature extraction | Raw data processing and biomarker quantification | Must be validated alongside hardware components |
Implementation Considerations for EDC Exposure Research
Successful deployment of wearable sensors in EDC research requires addressing several unique considerations:
Exposure Route Alignment: Select sensors and placement strategies aligned with primary EDC exposure routes. For dietary exposure monitoring, consider gastrointestinal response markers; for inhalation exposures, consider respiratory parameters; for dermal absorption, consider local skin response biomarkers [60].
Temporal Resolution Matching: Ensure sensor measurement frequency aligns with the pharmacokinetics of target EDCs and their physiological effects. Many EDCs exhibit complex, non-linear dose-response relationships that require high-resolution data to characterize properly [60].
Multimodal Data Integration: Develop strategies to integrate wearable sensor data with complementary exposure metrics, such as environmental monitoring data, food diaries, or product use inventories, to create comprehensive exposure assessments [60].
Participant Burden Management: Given the need for long-term monitoring to capture intermittent EDC exposures and chronic effects, prioritize wearable devices with minimal participant burden through comfortable designs, intuitive operation, and minimal maintenance requirements [61].
Wearable sensors and real-time data capture technologies represent a paradigm shift in exposure science research, offering unprecedented capabilities to monitor EDC exposures and their health effects continuously and in real-world contexts. These technologies enable researchers to move beyond traditional snapshot exposure assessments to capture dynamic relationships between EDC exposure through various routes (food, air, skin) and physiological responses.
The successful implementation of these technologies requires careful consideration of sensor selection, validation protocols, data management infrastructure, and analytical approaches. As the field advances, emerging capabilities in non-invasive biosensing, smart fabrics, and AI-driven analytics promise to further enhance our ability to delineate the complex relationships between EDC exposure and health outcomes across different life stages and populations.
For researchers, embracing these technologies requires interdisciplinary collaboration across exposure science, sensor engineering, data analytics, and clinical research. By leveraging the frameworks and methodologies outlined in this technical guide, research professionals can harness the power of direct data capture to advance our understanding of EDC impacts and develop more effective strategies for exposure mitigation and health protection.
Dose-Response Assessment and the Challenge of Non-Linear Relationships
The paradigm of dose-response assessment is undergoing a fundamental transformation, moving beyond traditional linear models toward sophisticated frameworks that account for non-linear relationships. This shift is particularly critical when assessing Endocrine Disrupting Chemicals (EDCs), where non-monotonic responses complicate risk evaluation. Evidence indicates that EDCs, including phthalates, bisphenol A (BPA), and per- and polyfluoroalkyl substances (PFAS), can disrupt reproductive health, alter metabolic function, and affect developmental processes at unexpectedly low doses. This whitepaper examines the mechanistic drivers of non-linearity—such as receptor saturation, metabolic detoxification, and adaptive stress responses—and provides researchers with advanced methodological approaches for designing studies, interpreting complex data, and incorporating these insights into chemical safety assessments. Special emphasis is placed on EDC exposure routes through food, air, and skin absorption within a broader thesis on their public health implications.
Historically, risk assessment for genotoxic chemicals operated on the assumption that even a single molecule could cause DNA damage, leading to a linear dose-response relationship at low doses. This "one-hit" model suggested that risk decreases proportionally with dose without a threshold [67]. However, increasing evidence challenges this paradigm across multiple chemical classes. Non-linear dose-responses suggest potential cellular tolerance to low levels of genotoxicants and EDCs, where adverse effects are not observed until a certain "practical threshold" is exceeded [68] [67].
The term "practical threshold" denotes a dose level below which no significant increase in adverse effect is observed, distinguishable from a theoretical absolute threshold. This concept is particularly relevant for EDCs, where non-linear relationships may result from the complex interplay between exposure timing, receptor affinity, and compensatory biological mechanisms [67] [69]. Understanding these non-linear patterns is essential for accurate risk assessment, especially given that regulatory frameworks traditionally relying on linear extrapolation may overestimate or underestimate risks at environmentally relevant exposure levels [70].
Mechanisms Driving Non-Linearity in Toxicological Responses
Biological Basis for Non-Linear Responses
Multiple cytoprotective mechanisms contribute to the observation of non-linear dose-response relationships, particularly at low exposure levels relevant to human environmental exposures:
- Cellular Repair Systems: DNA repair pathways, including base excision repair and nucleotide excision repair, effectively remove damage at low levels of genotoxic insult but become saturated at higher doses, leading to non-linear responses for alkylating agents and oxidants [68] [67].
- Metabolic Detoxification: Phase I and Phase II metabolic enzymes can effectively neutralize low concentrations of toxicants but become overwhelmed at higher doses, creating a threshold-like response [67].
- Receptor-Mediated Effects: For EDCs, receptor binding affinity, competition with endogenous hormones, and receptor down-regulation can produce non-monotonic dose-responses where effects at low doses differ from those at high doses [70] [36].
- Homeostatic Compensation: Cells maintain homeostasis through adaptive stress responses, antioxidant production, and apoptosis regulation, which can mitigate low-level insults but fail at higher exposure levels [68] [69].
These biological processes collectively enable cellular tolerance to low doses of many genotoxicants and EDCs with diverse modes of action, fundamentally challenging the universality of linear dose-response assumptions in toxicology [68].
Specific Mechanisms for Endocrine Disrupting Chemicals
EDCs exhibit particularly complex non-linear relationships due to their hormone-mimicking properties and impact on endocrine systems during critical developmental windows:
- Receptor Interactions: EDCs can bind to estrogen, androgen, and thyroid hormone receptors, either mimicking or blocking their functions. These interactions often show non-monotonic dose-responses due to receptor saturation and complex feedback mechanisms in the hypothalamic-pituitary-gonadal axis [70] [71].
- Critical Exposure Windows: Effects of EDCs vary significantly depending on life stage, with fetal development, infancy, and adolescence representing periods of heightened susceptibility. Exposure during these windows can cause permanent changes that manifest later in life, demonstrating non-linear temporal relationships between exposure and effect [60] [70].
- Epigenetic Modifications: Early-life EDC exposure can induce transgenerational effects through epigenetic mechanisms such as DNA methylation and histone modification, potentially contributing to obesity, reproductive disorders, and altered pubertal timing across generations [36].
- Oxidative Stress Pathways: Many EDCs, including PFAS and organochlorine pesticides, induce reactive oxygen species (ROS) formation, triggering adaptive responses at low doses but causing damage at higher exposures when antioxidant defenses are overwhelmed [67].
The following diagram illustrates key pathways through which EDCs entering via food, air, and skin absorption disrupt sexual development, particularly in adolescents:
Figure 1: EDC Impact on Adolescent Sexual Development. This diagram illustrates the multiple pathways through which endocrine-disrupting chemicals (EDCs), after entering the body via food, air, and skin absorption, disrupt normal adolescent sexual development, potentially leading to precocious puberty. Key mechanisms include disruption of the hypothalamic-pituitary-gonadal (HPG) axis, activation of growth signaling pathways, direct receptor interactions, and epigenetic modifications [70] [71].
Methodological Approaches for Assessing Non-Linear Relationships
Statistical Models and Data Analysis Techniques
Several advanced statistical approaches enable researchers to identify and characterize non-linear dose-response relationships:
- Benchmark Dose (BMD) Modeling: This approach identifies the dose that produces a predetermined change in response rate compared to background (benchmark response), typically 5-10%. The lower confidence limit of the BMD (BMDL) provides a point of departure for risk assessment, offering significant advantages over traditional NOAEL/LOAEL approaches [67].
- Nonlinear Mixed-Effects (NLME) Modeling: NLME models account for both fixed effects (population parameters) and random effects (individual variations), making them particularly valuable for analyzing sparse datasets common in human studies. These models are especially useful in pharmacokinetic studies where they can characterize parameter distributions across populations [72].
- Bilinear Hockey-Stick Models: These models identify a breakpoint (threshold dose) where the dose-response relationship changes slope, providing a statistical estimate of a practical threshold [67].
- Smoothing Regression Splines: Flexible spline-based methods can capture non-linear patterns without assuming a specific functional form, making them valuable for exploring complex dose-response shapes [67].
The following experimental workflow outlines key steps for establishing non-linear dose-response relationships for EDCs:
Figure 2: Experimental Workflow for Non-Linear Dose-Response Assessment. This workflow outlines the key stages in designing and conducting studies to identify non-linear dose-response relationships and potential thresholds for EDCs and other chemicals [68] [67].
Experimental Design Considerations
Proper study design is crucial for detecting and characterizing non-linear dose-response relationships:
- Dose Selection and Spacing: Studies should include multiple dose levels with appropriate spacing to detect potential thresholds. Including very low environmentally relevant doses is essential for EDC assessment where non-monotonic responses are common [67].
- Temporal Factors: The timing and duration of exposure must be carefully considered, particularly for EDCs where effects may manifest long after exposure, especially during critical developmental windows [70].
- Model System Selection: In vitro studies are particularly valuable for elucidating mechanisms of low-dose protection, while in vivo experiments are most appropriate for deriving a safe dose for human health risk assessment [68].
- Endpoint Specificity: Different endpoints (e.g., DNA adducts vs. chromosomal aberrations vs. mutations) may demonstrate different dose-response relationships within the same study, requiring careful endpoint selection based on assessment goals [67].
Case Studies and Chemical-Specific Applications
Endocrine Disrupting Chemicals
Research on EDCs provides compelling examples of non-linear dose-response relationships with significant implications for risk assessment:
- Phthalates: Epidemiological studies demonstrate consistent associations between phthalate exposure and obesity in children, with mixed results in adults, suggesting age-dependent susceptibility and potential non-linear relationships. DEHP exposure has been shown to disrupt the IGF-1/PI3K/Akt/mTOR signaling pathway, leading to altered hypothalamic development and precocious puberty in animal models [36] [71].
- Bisphenol A (BPA): BPA exhibits non-monotonic dose-responses, particularly for endocrine-sensitive endpoints. Low-dose BPA exposure promotes adipogenesis and disrupts lipid and glucose metabolism, contributing to obesity and metabolic disorders [36].
- PFAS Compounds: Exposure to per- and polyfluoroalkyl substances correlates with altered lipid profiles and increased adiposity, with evidence of transgenerational effects. Women with the highest combined exposure to pesticides and phthalates experience menopause 1.9-3.8 years sooner, indicating that EDCs lead to shorter reproductive lifespans in a dose-dependent manner [70].
- Pesticides: Organochlorine pesticides and other EDC pesticides have been linked to earlier breast development, infertility, polycystic ovary syndrome (PCOS), and earlier menopause, with effects observed at low environmental exposure levels [70].
Table 1: Non-Linear Dose-Response Characteristics of Selected Endocrine Disrupting Chemicals
| Chemical Class | Key Health Effects | Evidence for Non-Linearity | Proposed Mechanisms |
|---|---|---|---|
| Phthalates (e.g., DEHP) | Precocious puberty, reduced sperm count, obesity | Non-monotonic effects on reproductive development; age-dependent susceptibility | Disruption of HPG axis; IGF-1/PI3K/Akt/mTOR pathway alteration; oxidative stress [36] [71] |
| Bisphenol A (BPA) | Breast and prostate cancer, metabolic disorders, infertility | Non-monotonic dose-response for multiple endocrine endpoints | Estrogen receptor binding; adipogenesis promotion; lipid/glucose metabolism disruption [36] |
| PFAS | Altered lipid metabolism, early menopause, thyroid dysfunction | Transgenerational effects; altered associations at different exposure levels | PPAR activation; thyroid hormone disruption; epigenetic modifications [70] [36] |
| Organochlorine Pesticides | PCOS, infertility, hormone-dependent cancers | Differential effects at high vs. low doses; windows of susceptibility | Estrogen receptor binding; steroidogenesis disruption; oxidative stress [70] |
Genotoxic Compounds
Non-linear dose-responses have also been documented for various genotoxic compounds, informed by understanding their mode of action:
- Alkylating Agents: Ethyl methanesulfonate (EMS) and methyl methanesulfonate (MMS) show strongly non-linear dose-responses for in vivo mutagenicity, with demonstrated practical thresholds due to efficient DNA repair at low doses [67].
- Oxidants: Reactive oxygen species (ROS) demonstrate non-linear relationships due to the presence of adaptive responses and overlapping antioxidant defense systems that provide protection at low-level exposures [67].
- Aneugens: Chemicals that cause numerical chromosome abnormalities typically exhibit clear thresholds, as a critical number of target sites (e.g., tubulin molecules) must be impaired before improper chromosome segregation occurs [67].
- Topoisomerase Inhibitors: These compounds often show thresholded responses due to the requirement for sufficient enzyme inhibition before DNA cleavage events accumulate to pathogenic levels [67].
Table 2: Statistical Methods for Analyzing Non-Linear Dose-Response Relationships
| Method | Application | Advantages | Limitations |
|---|---|---|---|
| Benchmark Dose (BMD) Modeling | Point of departure identification for risk assessment | Uses all experimental data; accounts for study design and sample size; provides confidence intervals | Model dependency; requires multiple dose groups with adequate sample sizes [67] |
| Nonlinear Mixed-Effects (NLME) | Population pharmacokinetics/pharmacodynamics; sparse data settings | Accounts for between- and within-subject variability; suitable for unbalanced and sparse data | Computational complexity; requires specialized software [72] |
| Hockey-Stick Models | Threshold detection | Intuitive interpretation; directly estimates threshold dose | Oversimplification of complex biological responses; sensitive to dose selection [67] |
| Smoothing Regression Splines | Exploratory analysis of complex dose-response shapes | Flexibility to capture various functional forms; minimal assumptions about relationship | Risk of overfitting; limited inferential framework [67] |
Research Implications and Toolkit
The Scientist's Toolkit: Essential Research Reagents and Methods
Research on non-linear dose-response relationships for EDCs requires specialized experimental approaches and analytical tools:
Table 3: Essential Research Reagents and Methods for EDC Dose-Response Studies
| Reagent/Method | Function/Application | Key Considerations |
|---|---|---|
| Gene Knockout/Transgenic Models | Elucidate specific pathways in low-dose protection; test mechanistic hypotheses | Enables identification of critical pathways; allows determination of mode of action [68] |
| Chemical Modulators of Key Effectors | Inhibit or activate specific pathways to assess impact on dose-response | Demonstrates causal relationships; identifies metabolic and repair pathways [68] [67] |
| Biomarker Assays (e.g., DNA adduct quantification by mass spectrometry) | Measure internal dose, early biological effects, and susceptibility | High sensitivity for low-dose detection; distinguishes primary DNA lesions from fixed mutations [67] |
| High-Throughput Technologies (e.g., flow cytometry) | Enhance statistical power of genotoxicity assays through rapid cell analysis | Enables detection of rare events; improves quantitative assessment of dose-response relationships [67] |
| Transgenic Rodent Mutation Assays (e.g., MutaMouse, Big Blue) | Assess in vivo mutagenicity across multiple tissues | Provides critical data on fixed mutations; allows organ-specific evaluation of dose-response [67] |
| Non-Linear Mixed-Effects Modeling Software (e.g., MonolixSuite) | Analyze complex pharmacokinetic/pharmacodynamic data | Handles sparse, unbalanced data; estimates population and individual parameters simultaneously [72] [73] |
Implications for Risk Assessment and Regulatory Science
The recognition of non-linear dose-response relationships has profound implications for chemical risk assessment and regulatory decision-making:
- Threshold of Toxicological Concern: Evidence of practical thresholds for certain modes of action supports the use of threshold-based approaches for risk assessment of many EDCs and genotoxicants, potentially replacing default linear extrapolation [67].
- Cumulative Risk Assessment: Regulatory frameworks must account for cumulative effects of mixed EDC exposures, as current approaches often fail to consider combined lifetime exposure effects, especially during developmentally sensitive periods [70].
- Sensitive Population Protection: Early-life exposure to EDCs during critical developmental windows has been shown to increase obesity and reproductive disorder risk later in life, necessitating special protection measures for vulnerable populations [36].
- Chemical Testing Strategies: Study designs should incorporate a sufficient number of appropriately spaced dose levels to detect potential non-linearities, with emphasis on including environmentally relevant low doses [67].
The assessment of non-linear dose-response relationships represents a critical advancement in toxicological sciences, with particular relevance for Endocrine Disrupting Chemicals that exhibit complex, non-monotonic response patterns. Moving beyond traditional linear models requires robust mechanistic data, sophisticated statistical approaches, and study designs capable of detecting thresholded responses. Evidence from genotoxicants and EDCs indicates that cytoprotective mechanisms—including DNA repair, metabolic detoxification, and receptor dynamics—frequently produce non-linear dose-response relationships with practical thresholds. For EDCs specifically, the timing of exposure appears to be as critical as the dose, with early-life exposures producing permanent effects that manifest across the lifespan. Future research should focus on elucidating the full spectrum of obesogens and reproductive toxicants, their mechanisms of action, and implications for disease risk across generations. This knowledge will inform evidence-based preventive strategies and public health interventions aimed at addressing the growing burden of disease associated with EDC exposure.
Navigating Complexities: Risk Assessment, Mixture Effects, and Intervention Strategies
Human Health Risk Assessment (HHRA) is a systematic, scientific process used to estimate the nature and likelihood of adverse health effects in humans who may be exposed to hazardous agents in their environment [74]. This methodology is fundamental to public health decision-making, providing a structured framework for evaluating the potential risks posed by chemical, physical, and biological stressors. For researchers investigating Endocrine Disrupting Chemicals (EDCs), the HHRA framework offers a critical pathway for translating experimental data into evidence-based conclusions about human health risks. EDCs represent a particularly challenging class of compounds as they interfere with hormone action and have been associated with diverse health outcomes including cardiometabolic diseases, neurological disorders, and various cancers [3] [75] [8]. The assessment process enables scientists to move from hazard identification to quantitative risk characterization, thereby informing regulatory standards and public health policies aimed at reducing exposure to these concerning environmental contaminants.
The Four-Step Framework for Human Health Risk Assessment
The United States Environmental Protection Agency (EPA) has established a standardized four-step process for conducting human health risk assessments, which begins with a comprehensive planning and scoping phase [74] [76]. This systematic approach ensures that risk assessments are thorough, transparent, and scientifically defensible.
Planning and Scoping
Before initiating the technical assessment, risk assessors engage in detailed planning to define the assessment's purpose, scope, and methodological approach [74] [76]. This critical preliminary phase involves dialogue between risk managers, risk assessors, and other stakeholders to establish clear objectives and boundaries for the assessment. Key considerations during planning include identifying the specific populations at risk (general population, susceptible subgroups, or life stages such as children or pregnant women), the environmental hazards of concern (single chemicals, mixtures, or specific classes like EDCs), exposure sources (point sources, non-point sources, or natural sources), and exposure pathways (air, water, soil, food, or consumer products) [76]. For EDC research, this phase typically includes explicit consideration of unique susceptibility windows (e.g., developmental stages) and non-traditional dose-response relationships that may not exhibit conventional monotonic patterns [75].
Step 1: Hazard Identification
Hazard identification is the foundational step that determines whether exposure to a stressor can cause increased incidence of specific adverse health effects and whether these effects are likely to occur in humans [76]. For EDCs, this involves examining the scientific evidence linking specific chemicals to endocrine disruption and associated health outcomes. Researchers evaluate multiple data sources, including statistically controlled clinical studies on humans (when available), epidemiological studies of human populations, and experimental studies using animal models or in vitro systems [76]. The process employs toxicokinetic studies (how the body absorbs, distributes, metabolizes, and eliminates chemicals) and toxicodynamic studies (the effects chemicals have on the human body) to understand the biological mechanisms underlying potential health effects [76]. A key component is developing a weight of evidence characterization that synthesizes all available data to categorize the strength of the link between a chemical and adverse health outcomes. For potential carcinogens, EPA practice includes analysis of mode of action—the sequence of key events starting with interaction of an agent with a cell and proceeding through functional changes resulting in cancer formation [76].
Step 2: Dose-Response Assessment
The dose-response assessment quantitatively characterizes the relationship between the magnitude of exposure and the probability of occurrence of the adverse health effects identified in Step 1 [76]. This step documents how the likelihood and severity of health effects are related to the amount and conditions of exposure to an environmental agent. Typically, as the dose increases, the measured response also increases, though the specific shape of the dose-response relationship varies depending on the agent, the type of response (e.g., tumor incidence, metabolic disruption), and the subject (human or animal) [76]. A critical aspect of this step is identifying the critical effect—the adverse effect (or its precursor) that occurs at the lowest dose—with the assumption that preventing this effect will prevent all other adverse effects at higher doses [76]. For EDCs, this assessment must consider potential non-monotonic dose responses and heightened susceptibility during developmental windows [75]. When human data are unavailable, researchers must extrapolate from high-dose animal studies to lower doses relevant to human exposure, introducing uncertainty that must be explicitly acknowledged and quantified.
Step 3: Exposure Assessment
The exposure assessment examines what is known about the frequency, timing, and levels of contact with the environmental stressor [74]. This step characterizes the nature of human exposure, including identifying exposed populations, exposure pathways (air, water, soil, food, consumer products), exposure routes (inhalation, ingestion, dermal absorption), and the magnitude, duration, and frequency of exposure [76]. For EDCs, which contaminate numerous environmental media and consumer products, exposure assessment is particularly complex. Humans are exposed to EDCs through diverse sources including plastic containers, food packaging, cosmetics, personal care products, electronics, insulation, and pesticides [75] [11]. Exposure occurs through multiple pathways: diet, inhalation, and skin contact due to environmental contamination of water, soil, and air [3]. Prenatal exposure occurs via the placenta, and postnatal exposure continues through breastfeeding [3] [77]. Sophisticated exposure assessment methods for EDCs include human biomonitoring (HBM) to measure chemicals or their metabolites in biological samples like urine, blood, and breast milk [78]. The European Partnership for the Assessment of Risks from Chemicals (PARC) employs aligned HBM studies across multiple countries to assess chemical exposure in different age groups, providing comprehensive exposure data for risk assessment [78].
Step 4: Risk Characterization
Risk characterization integrates information from hazard identification, dose-response assessment, and exposure assessment to develop a comprehensive estimate of the nature and magnitude of risk [74]. This final step includes two major components: risk estimation, which compares the estimated exposure levels with the dose-response data to quantify likelihood of adverse effects, and risk description, which provides information needed to interpret the risk results [74]. The risk description includes discussion of whether harmful effects are expected in the populations of concern, relevant qualitative comparisons, and how uncertainties (data gaps and natural variation) might affect the assessment [74]. For EDCs, risk characterization must explicitly address uncertainties related to non-traditional dose-response curves, effects of mixtures, and sensitive subpopulations [3] [75]. The output of this step provides risk managers with the scientific basis for regulatory decisions, such as establishing acceptable exposure limits or implementing risk mitigation strategies.
Table 1: Key Questions Addressed at Each Stage of HHRA Planning
| Assessment Stage | Core Questions | EDC-Specific Considerations |
|---|---|---|
| Population Identification | Who/what/where is at risk? General population or susceptible subgroups? [76] | Children, pregnant women, occupational groups with high exposure [74] [78] |
| Hazard Characterization | What is the environmental hazard? What are the health effects? [76] | Endocrine disruption mechanisms; cardiometabolic, neurological, or reproductive effects [3] [75] [8] |
| Exposure Analysis | Where do hazards come from? How does exposure occur? [76] | Multiple sources: plastics, food packaging, cosmetics, electronics; routes: ingestion, inhalation, dermal [75] [11] |
| Dosimetry | What does the body do with the environmental hazard? [76] | Toxicokinetics: absorption, distribution, metabolism, excretion; bioaccumulation potential [76] |
| Temporal Factors | How long does it take for effects to appear? Does timing of exposure matter? [76] | Critical windows of susceptibility (developmental stages); chronic vs. acute exposure [74] [75] |
Special Considerations for Endocrine Disrupting Chemicals (EDCs)
EDCs present unique challenges for risk assessment due to their specific mechanisms of action, non-traditional dose-response relationships, and heightened effects during sensitive developmental windows. Understanding these special considerations is essential for applying the HHRA framework effectively to this class of chemicals.
Mechanisms of Endocrine Disruption
EDCs interfere with the endocrine system through multiple molecular mechanisms that complicate traditional risk assessment approaches. These substances can mimic natural hormones (agonistic action), block hormone receptors (antagonistic action), alter hormone synthesis and metabolism, or modify hormone receptor expression and function [75] [11]. A prominent example is Bisphenol A (BPA), which has structural features that enable it to bind to estrogen receptors and disrupt lipid metabolism, inducing cardiac edema in zebrafish embryos and triggering ventricular arrhythmias in female rat hearts [11]. EDCs can also disrupt thyroid function, with significant consequences for brain development and metabolic regulation [3]. The complex mechanisms of endocrine disruption mean that EDCs may produce effects at extremely low doses, exhibit non-monotonic dose-response curves where effects occur at low but not high doses, and cause delayed effects that manifest long after exposure has ceased [75]. These characteristics challenge the traditional toxicological principle that "the dose makes the poison" and require specialized testing approaches within the HHRA framework [74].
Sensitive Subpopulations and Life Stages
Children and developing fetuses represent particularly vulnerable populations for EDC exposure due to several physiological and behavioral factors [74]. Pound for pound, children breathe more air, drink more water, and eat more food than adults, resulting in proportionally greater exposure to environmental toxicants [74]. Their behavior patterns, such as playing close to the ground and frequent hand-to-mouth activity, further increase exposure potential [74]. Critically, children may be more vulnerable to environmental hazards because their organ systems are still developing, often making them less able to metabolize, detoxify, and excrete toxins [74]. For pollutants that act as developmental toxicants, the same dose that may pose little or no risk to an adult can cause drastic effects in a developing fetus or child [74]. Methyl mercury provides one example of a chemical that is much more toxic early in life [74]. These concerns led to Executive Order 13045 in 1997, directing all federal agencies to make it a high priority to identify and assess environmental health risks that may disproportionately affect children [74].
Table 2: Common EDCs, Their Sources, and Major Health Concerns
| EDC or Class | Typical Use/Source | Major Health Concerns | Detection Frequency |
|---|---|---|---|
| Bisphenols (BPA, BPS) | Polycarbonate plastics, epoxy resins (food/beverage containers, can linings, toys) [75] [11] | Cardiometabolic disease, reproductive effects, developmental toxicity [75] [8] [11] | BPA detected in >50% of breast milk samples in Chinese study [77] |
| Phthalates | Plasticizers in plastics; food packaging, cosmetics, fragrances, toys, medical tubing [75] [11] | Impaired glucose metabolism, childhood obesity, male reproductive tract abnormalities [75] [8] | Widely detected in human biomonitoring studies [78] |
| Per- and polyfluoroalkyl substances (PFAS) | Firefighting foams, surface protectants (fabrics, food packaging, nonstick cookware) [75] [11] | Thyroid disruption, immune suppression, dyslipidemia, reduced vaccine response [75] [8] | Persistent in environment and human tissues; monitored in PARC [78] |
| Triclosan and triclocarban | Antimicrobial agents in personal care and cleaning products (soaps, toothpastes, detergents) [75] [11] | Thyroid hormone disruption, antibiotic resistance, potential cardiometabolic effects [75] | Detected in >50% of breast milk samples [77] |
| Parabens (MP, EP, PP) | Preservatives in cosmetics, personal care products, pharmaceuticals [77] | Estrogenic activity, potential breast cancer association, reproductive effects [77] | MP detected in 0.37 ng/mL median concentration in breast milk [77] |
| Polychlorinated biphenyls (PCBs) | Electrical equipment (transformers, capacitors); banned but persistent [75] [11] | Neurodevelopmental deficits, cognitive impairment, potential carcinogens [75] [8] | Persistent in environment despite bans |
Advanced Methodologies and Emerging Approaches
The field of human health risk assessment is rapidly evolving with new scientific approaches that enhance our ability to evaluate EDC risks, particularly given the limitations of traditional methods.
New Approach Methodologies (NAMs)
New Approach Methodologies (NAMs) represent innovative testing strategies that aim to modernize chemical risk assessment by addressing limitations of traditional animal studies [79]. NAMs include in vitro methods, computational toxicology approaches, high-throughput screening (HTS) assays, genomics, and other advanced techniques that can provide human-relevant toxicity data while reducing animal testing [79]. These methodologies are particularly valuable for EDC assessment because they can efficiently screen large numbers of chemicals for endocrine activity, identify mechanisms of action, and evaluate species-specific responses. However, a mixed-methods study investigating the perspectives of human health risk assessors found heterogeneous familiarity and use of specific NAMs across regulatory agencies, industry, and academia [79]. While approaches like Quantitative Structure-Activity Relationships (QSARs) are well-known and used, other methods such as -omics approaches are seldom used in regulatory contexts [79]. Key barriers to NAM implementation include validity concerns, limited regulatory acceptance, and need for standardized guidance documents [79]. The European Partnership for the Assessment of Risks from Chemicals (PARC) is working to address these challenges by developing and implementing innovative tools and methods for chemical risk assessment [78].
Human Biomonitoring and Exposure Science Innovations
Advanced exposure assessment methods are critical for characterizing real-world EDC exposures, particularly given the complex mixture of chemicals encountered in daily life. Human Biomonitoring (HBM) has emerged as an essential tool for assessing integrated exposure to environmental pollutants by analyzing chemicals or their metabolites in biological samples like urine, blood, breast milk, and hair [78]. HBM studies provide direct measures of internal exposure from all sources and routes, offering significant advantages over environmental monitoring alone. The PARC initiative is conducting EU-wide aligned HBM studies to assess chemical exposure across different age groups: children (6-11 years) in 16 countries, adolescents (12-17 years) in 12 countries, and adults (18-39 years) in 20 countries [78]. These studies employ innovative techniques including non-target screening (NTS) and suspect screening to identify previously unrecognized chemical exposures, self-sampling methods, non-invasive sampling, micro-sampling, and exposure assessment through personal sensors including wristbands and mobile apps [78]. For EDCs with short half-lives but continuous exposure (creating "pseudo-persistence"), these advanced methods are particularly valuable for capturing exposure patterns that traditional approaches might miss [11].
Mixture Risk Assessment
Humans are invariably exposed to complex mixtures of EDCs rather than single compounds, creating significant challenges for risk assessment. The combination of multiple EDCs may produce additive, synergistic, or antagonistic effects that cannot be predicted from single-chemical evaluations [75]. Research on 13 EDCs in pharmaceuticals and personal care products found that cumulative endocrine disruption risk intensity followed the order MP > TCS > BPA > EP > PP, highlighting the importance of considering combined effects [77]. Across 20 Chinese cities, 0%-40% of infants had a hazard index (HI) exceeding 1 when exposed to multiple EDCs through breast milk, indicating potential health concerns from combined exposures [77]. To address these challenges, researchers are developing mixture-centered risk assessment strategies that integrate epidemiological and experimental evidence to characterize risks from EDC mixtures [75]. The PARC initiative is implementing a monitoring cycle that includes prioritization of chemicals, design of monitoring studies, monitoring activities, data analysis, and feedback mechanisms to support mixture risk assessment [78]. This approach includes pilot studies on prioritized substance groups including per- and polyfluoroalkyl substances (PFAS) and EDCs to establish baseline environmental concentrations and characterize exposure routes [78].
Experimental Protocols for EDC Risk Assessment
Implementing the HHRA framework for EDCs requires specific methodological approaches tailored to the unique properties of these chemicals. The following section details key experimental protocols relevant to EDC risk assessment.
Hazard Identification Protocols
In Vitro Receptor Binding Assays
Purpose: To screen chemicals for potential endocrine activity by measuring their ability to bind hormone receptors. Methodology:
- Isolate or express specific human hormone receptors (estrogen receptors α and β, androgen receptor, thyroid receptor, etc.)
- Incubate receptors with test chemicals at varying concentrations
- Use competitive binding assays with labeled natural hormones to quantify displacement
- Calculate relative binding affinity compared to native hormones Data Interpretation: Chemicals exhibiting significant receptor binding at environmentally relevant concentrations proceed to more comprehensive toxicological evaluation.
Transcriptional Activation Assays
Purpose: To determine whether receptor binding translates to functional changes in gene expression. Methodology:
- Transfert mammalian cells with reporter gene constructs (e.g., luciferase) under control of hormone-responsive elements
- Expose to test chemicals across concentration range (typically 10^-12 to 10^-5 M)
- Measure reporter gene activity after 24-48 hours exposure
- Include appropriate controls (vehicle, positive controls with native hormones) Data Interpretation: Dose-response curves indicate potency and efficacy compared to native hormones; note non-monotonic responses.
Dose-Response Assessment Protocols
Animal Testing for Endocrine-Sensitive Endpoints
Purpose: To characterize adverse effects of EDCs on endocrine-sensitive tissues and functions at different exposure levels. Methodology:
- Study Design: Implement OECD Test Guidelines 440 (Utertrophic Bioassay) or 441 (Hershberger Bioassay)
- Dosing: Administer at least three dose levels plus vehicle control; include reference estrogen/testosterone as positive control
- Exposure Window: Critical life stages (developmental, perinatal, pubertal) depending on endocrine endpoint of interest
- Endpoint Measurement: Organ weights (uterus, prostate, etc.), histopathology, serum hormone levels, gene expression
- Statistical Analysis: Determine benchmark doses (BMD) and no-observed-adverse-effect-levels (NOAELs) using appropriate models Special Considerations for EDCs: Test wider dose range than traditional toxicology studies; include very low doses to detect potential non-monotonic responses.
Exposure Assessment Protocols
Human Biomonitoring for EDCs
Purpose: To measure internal concentrations of EDCs and their metabolites in human populations. Methodology:
- Sample Collection: Collect biological matrices (urine, serum, breast milk) using protocols that prevent contamination
- Chemical Analysis: Utilize LC-MS/MS for precise quantification of EDCs and metabolites
- For breast milk analysis: Use ultra-high-performance liquid chromatography-triple quadrupole mass spectrometry [77]
- Apply solid-phase extraction for sample cleanup and concentration
- Use stable isotope-labeled internal standards for quantification
- Quality Assurance/Quality Control: Implement rigorous QA/QC including blanks, duplicates, and reference materials
- Data Normalization: Adjust for creatinine (urine) or lipid content (serum, breast milk) to account for matrix variations Application: The PARC initiative employs aligned HBM protocols across multiple European countries to ensure comparable data on EDC exposure [78].
Table 3: Research Reagent Solutions for EDC Risk Assessment
| Reagent/Method | Function in EDC Assessment | Example Application | Regulatory Status |
|---|---|---|---|
| Recombinant Human Hormone Receptors | Screening for receptor binding affinity | Initial prioritization of chemicals with endocrine activity | Accepted screening tool in EPA EDSP [75] |
| Reporter Gene Assays | Assessment of transcriptional activation | Mechanism-specific evaluation of estrogenic, androgenic, or thyroid activity | Validated OECD test guidelines available |
| Stable Isotope-Labeled Standards | Internal standards for analytical quantification | Precise measurement of EDCs in biological and environmental matrices | Essential for HBM studies in PARC [78] |
| CALUX Assays | High-throughput screening for receptor-mediated activity | Rapid screening of environmental samples for endocrine activity | Used in combination with chemical analysis in EU monitoring [78] |
| CRISPR-Modified Cell Lines | Pathway-specific mechanistic studies | Evaluation of specific endocrine pathways in human-relevant systems | Emerging tool not yet standardized for regulatory use [79] |
| Organ-on-a-Chip Models | Human-relevant tissue response assessment | Evaluation of EDC effects on specialized tissues (mammary, testis, thyroid) | Promising NAM not yet validated for regulatory decisions [79] |
Data Analysis and Interpretation
Proper analysis and interpretation of data are crucial for deriving meaningful conclusions from EDC risk assessments. This section outlines key analytical approaches and their application within the HHRA framework.
Quantitative Risk Characterization
Risk characterization for EDCs integrates data from hazard identification, dose-response assessment, and exposure assessment to quantify potential health risks. For threshold effects, risk assessors typically calculate hazard indices (HI) or margin of exposure (MOE) values [77]. The hazard index approach sums the ratios of exposure to reference values for multiple chemicals acting through similar mechanisms. Research on EDCs in breast milk found that 0%-40% of infants across 20 Chinese cities had HIs exceeding 1, indicating potential concern from combined exposures [77]. For cumulative risk assessment of EDCs with similar mechanisms, the toxicological priority index (ToxPi) incorporates population exposure data with toxicity potency metrics to visualize and prioritize risks from multiple chemicals [77]. For non-threshold effects like carcinogenicity, risk assessors use low-dose extrapolation models to estimate excess cancer risk at environmental exposure levels. The European Food Safety Authority (EFSA) and EPA have developed specific guidance for assessing susceptibility from early-life exposure to carcinogens, which is particularly relevant for EDCs with genotoxic potential [74].
Uncertainty and Variability Analysis
Comprehensive uncertainty analysis is essential for transparent EDC risk assessment. Key sources of uncertainty include intra- and inter-species extrapolation, differences in exposure timing and duration, and variability in human susceptibility [76]. For EDCs, additional uncertainties arise from potential non-monotonic dose responses, effects of chemical mixtures, and sensitive subpopulations [75]. Risk assessments should explicitly characterize these uncertainties using qualitative descriptions and, when possible, quantitative techniques such as probabilistic modeling or Monte Carlo simulation. Bayesian approaches are particularly valuable for integrating evidence from multiple sources (epidemiology, animal studies, in vitro data) while explicitly accounting for uncertainty in each evidence stream. The weight-of-evidence approach used by EPA and other regulatory agencies provides a structured framework for evaluating the quality and consistency of the entire body of evidence rather than relying on single studies [76] [75].
Diagram 1: HHRA Framework for EDCs - This diagram illustrates the four-step Human Health Risk Assessment process as applied to endocrine disrupting chemicals, highlighting key data sources and EDC-specific considerations at each stage.
Diagram 2: EDC Exposure-to-Risk Assessment Pathway - This diagram maps the complete pathway from EDC sources through environmental media, exposure pathways, assessment methods, and final risk characterization outcomes, highlighting the complexity of EDC exposure assessment.
The Human Health Risk Assessment framework provides a rigorous, systematic approach for evaluating potential health risks from exposure to endocrine disrupting chemicals. While the standardized four-step process (hazard identification, dose-response assessment, exposure assessment, and risk characterization) offers a robust foundation, assessing EDCs requires special consideration of their unique properties, including non-monotonic dose responses, sensitivity during developmental windows, and mixture effects. Advanced methodologies such as human biomonitoring, non-target screening, and New Approach Methodologies are enhancing our ability to characterize EDC risks more accurately and efficiently. The growing body of evidence linking EDC exposure to diverse health outcomes including cardiometabolic diseases, neurological disorders, and various cancers underscores the importance of applying this rigorous assessment framework to inform evidence-based decision-making [75] [8] [11]. As research continues to evolve, particularly regarding the effects of real-world mixtures and sensitive subpopulations, the HHRA framework will remain essential for translating scientific findings into protective public health policies.
Endocrine-disrupting chemicals (EDCs) are exogenous substances that interfere with the normal function of the endocrine system, leading to adverse health effects in intact organisms, their progeny, or subpopulations [80]. These synthetic chemicals contaminate indoor and outdoor air, water, and food, creating nearly unavoidable exposure in daily life through three primary routes: food ingestion, respiratory pathways, and skin absorption [13] [81]. The reproductive system represents the most affected human system from EDC exposure, with many EDCs acting as estrogen mimics or anti-estrogen compounds [13]. Importantly, EDCs do not follow the classic toxicological principle of "the dose makes the poison," as they demonstrate non-monotonic dose-response curves where effects may be more pronounced at low doses than at high doses, and their impacts can be potentiated when multiple EDCs combine in "cocktail" effects [81].
The timing of exposure is critically important, with fetal development representing a period of particular vulnerability. During gestation, EDCs can cross the placental barrier and interfere with developmental processes, while in postnatal life, exposure continues through breast milk, contaminated food and water, inhalation of polluted air, and dermal absorption from personal care products and household items [70]. The lipophilic nature of many EDCs enables them to accumulate in adipose tissue for months or even years, meaning their effects may manifest long after the actual exposure occurs [81]. This combination of widespread environmental contamination, multiple exposure routes, and sensitive developmental windows creates significant public health challenges that require sophisticated research approaches to fully understand.
Low-Dose Effects of EDCs
Mechanisms of Low-Dose Action
The low-dose effects of EDCs present a significant challenge to traditional toxicological risk assessment. Unlike conventional toxicants, EDCs can produce substantial effects at doses far below those used in traditional toxicity testing [28]. These effects occur through multiple interconnected mechanisms, including nuclear receptor interactions, epigenetic modifications, and oxidative stress pathways. EDCs can bind to hormone receptors such as estrogen, androgen, thyroid, and glucocorticoid receptors, acting as agonists or antagonists at remarkably low concentrations [82] [83]. This receptor binding can trigger conformational changes that alter co-activator recruitment and downstream gene expression, even at concentrations previously considered biologically insignificant.
At the molecular level, EDCs interfere with hormone synthesis, metabolism, transport, and signaling pathways [84]. They can bind to hormone receptors, modify gene expression, and disrupt the delicate feedback loops responsible for regulating hormone production and release. These disruptions can result in abnormal hormone levels and perturbations in developmental processes, particularly during critical windows of vulnerability such as fetal development, early childhood, and adolescence [13]. The non-monotonic dose-response curves observed with many EDCs occur because they interact with hormones and activate their receptors in a nonlinear fashion, leading to U-shaped or inverted U-shaped curves rather than traditional sigmoidal dose-response relationships [80].
Table 1: Characteristics of Low-Dose EDC Effects
| Characteristic | Traditional Toxicants | Endocrine Disruptors |
|---|---|---|
| Dose-Response Relationship | Monotonic | Non-monotonic (U-shaped or inverted U-shaped) |
| Threshold Principle | Applies | Does not consistently apply |
| Sensitive Periods | Limited developmental windows | Expanded vulnerable periods throughout lifespan |
| Mechanisms of Action | Typically single pathway | Multiple interconnected pathways |
| Mixture Effects | Often additive | Potentiating "cocktail effects" |
| Latency of Effects | Usually short-term | May manifest years after exposure |
Experimental Evidence for Low-Dose Effects
Substantial experimental evidence demonstrates the reality and significance of low-dose EDC effects. For bisphenol A (BPA), urinary concentrations in the US population average approximately 6 µg/L, with highest exposures in bottle-fed children ranging from 0.01 to 13 µg/kg/day [80]. Despite these low exposure levels, numerous studies have documented effects on cancer development, metabolism, heart disease, fertility, and neurodevelopment [80]. The United States Environmental Protection Agency (US-EPA) safety level for BPA is set at 50 µg/kg/day, while the European Food Safety Authority's (EFSA) temporary tolerable daily intake was recently lowered to 0.2 ng/kg/day, reflecting growing concern about low-dose effects [80].
Laboratory studies reveal that low-dose EDC exposure during development can alter cellular processes fundamental to normal development, including differentiation, proliferation, apoptosis, and migration [83]. These disruptions at the cellular level can lead to abnormal organogenesis and tissue patterning, with consequences that may not become apparent until much later in life. For example, low-dose exposure to BPA has been shown to modify the timing of neuronal migration and neurogenesis in the developing brain, potentially contributing to neurobehavioral disorders [82]. These findings underscore the importance of investigating low-dose effects using sensitive experimental models and methodological approaches.
Transgenerational Impacts of EDCs
Epigenetic Mechanisms of Transgenerational Inheritance
The transgenerational effects of EDCs represent one of the most significant and concerning aspects of their toxicity profile. True transgenerational inheritance requires the transmission of phenotypic traits to generations that were never directly exposed to the EDC. When exposure occurs during pregnancy, the F3 generation represents the first truly non-exposed generation, as the F0 mother, F1 embryo, and F2 germline are all directly exposed [80]. The mechanisms underlying these transgenerational effects primarily involve epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNA expression that can be passed through the germline to subsequent generations [82] [83].
Emerging research suggests that the sperm genome is particularly poised for transgenerational transmission of epigenetic information. During sperm development, gene enhancers and promoters become accessible for transcription, and these activating motifs are also found in preimplantation embryos [82]. This means that DNA modifications associated with transcription factors during fertilization, in primordial germ cells, or during germ cell maturation may be passed to offspring. The Skinner laboratory's pioneering work demonstrated that exposure to the anti-androgenic fungicide vinclozolin during fetal germ cell migration (gestational days 10-15 in rats) – when DNA methylation is being re-established – can produce transgenerational effects on sperm viability and behavior persisting through the F3 generation and beyond [82].
Table 2: Epigenetic Mechanisms in EDC-Mediated Transgenerational Effects
| Epigenetic Mechanism | Process | Transgenerational Evidence |
|---|---|---|
| DNA Methylation | Addition of methyl groups to cytosine bases | Differential methylation patterns in sperm of F3 vinclozolin-lineage rats |
| Histone Modifications | Post-translational modification of histone proteins | Altered histone acetylation and methylation in germ cells |
| Non-coding RNAs | Regulation of gene expression by miRNA, siRNA | Sperm miRNA content alterations after EDC exposure |
| Chromatin Remodeling | Changes in chromatin structure and accessibility | Accessible promoters and enhancers in sperm genome |
Experimental Models and Transgenerational Phenotypes
Well-established experimental models have demonstrated transgenerational effects for several EDCs, including vinclozolin, bisphenol A, phthalates, and other compounds. In the classic vinclozolin transgenerational study design, rat dams are exposed during the period of fetal germ cell migration (gestational days 10-15), and offspring are bred through the F3 generation without additional exposure [82]. The F3 generation displays numerous phenotypic alterations, including decreased sperm motility and numbers, sex-specific behavioral changes, and altered gene expression patterns in the brain [82]. Notably, F3 females discriminate between control and vinclozolin-lineage males, preferring males without exposure history, while F3 males display decreased anxiety-like behavior and altered social novelty preferences [82].
Similar transgenerational effects have been observed with BPA exposure, which can promote both hypo- and hyper-DNA methylation patterns that may be transmitted to subsequent generations [82]. The transgenerational impacts of EDCs extend beyond reproductive parameters to include neurobehavioral disorders, metabolic diseases, and increased cancer susceptibility [81] [83]. These findings have profound implications for public health, as they suggest that current exposures may affect the health and development of multiple future generations, even if those generations encounter no direct EDC exposure.
Methodological Approaches and Experimental Protocols
Assessing Low-Dose Effects: Protocol Framework
Investigating the low-dose effects of EDCs requires carefully designed experimental protocols that account for their unique characteristics. A comprehensive assessment should include:
Animal Model Selection and Exposure Regimen: Rodent models (rats or mice) are typically used, with exposure occurring during critical developmental windows (gestation, lactation, puberty). Doses should include environmentally relevant concentrations (often in the ng/kg to μg/kg range) in addition to the higher doses used in traditional toxicology studies. For example, BPA studies might include doses of 0.25, 2.5, 25, and 250 μg/kg/day, compared to the EPA reference dose of 50 μg/kg/day [80]. Exposure should occur via relevant routes (oral, inhalation, dermal) depending on the chemical and research question.
Tissue Collection and Molecular Analysis: At endpoint, tissues of interest (brain, liver, reproductive organs, adipose tissue) should be collected for histological, molecular, and epigenetic analyses. This includes assessment of hormone receptor expression (ERα, ERβ, AR, TR), steroidogenic enzyme activity, and transcriptomic profiling. Special attention should be paid to epigenetic endpoints including DNA methylation patterns (via whole-genome bisulfite sequencing), histone modifications (ChIP-seq for H3K4me3, H3K27ac), and non-coding RNA expression [82] [83].
Statistical Considerations: Studies must be powered to detect non-monotonic dose-response relationships, which may require larger sample sizes than traditional toxicology studies. Statistical approaches should include tests for nonlinearity and careful consideration of multiple comparison issues in omics datasets.
Transgenerational Study Design: Protocol Framework
Transgenerational studies require multigenerational breeding schemes and careful experimental design:
Generational Breeding Scheme: The foundational protocol involves exposing pregnant F0 dams during critical windows of fetal germ cell development (e.g., E8-E15 in mice, E10-E15 in rats), then breeding their offspring (F1) to produce F2, and F2 to produce F3 generations without any additional exposure [82]. The F3 generation represents the first truly non-exposed generation for maternal lineage exposures. To assess paternal lineage contributions, F0 males are exposed before mating with naive females.
Phenotypic Assessment Across Generations: Each generation should be assessed for relevant phenotypic endpoints including reproductive parameters (sperm quality, ovarian follicle counts, fertility metrics), metabolic parameters (body weight, glucose tolerance, adiposity), neurobehavioral traits (anxiety-like behavior, social behaviors, cognitive function), and disease susceptibility [82]. Tissue collection should include germ cells (sperm, oocytes) for epigenetic analysis.
Epigenetic Mapping: Comprehensive epigenetic profiling should include whole-genome bisulfite sequencing of sperm and oocytes, histone modification mapping in germ cells, and assessment of non-coding RNA populations. Emerging techniques such as ATAC-seq can reveal changes in chromatin accessibility that may be transmitted across generations [82].
Transgenerational Inheritance Pathway
Signaling Pathways and Molecular Mechanisms
Key Pathways Disrupted by EDCs
EDCs interfere with numerous signaling pathways critical for development and homeostasis. The primary pathways affected include:
Nuclear Receptor Signaling: EDCs can directly bind to and activate or antagonize hormone receptors including estrogen receptors (ERα and ERβ), androgen receptor (AR), thyroid hormone receptors (TRα and TRβ), and peroxisome proliferator-activated receptors (PPARs) [82] [83]. BPA, for example, acts as an estrogen receptor agonist, leading to altered expression of estrogen-responsive genes [80]. The specific conformational changes induced by EDC binding can alter co-regulator recruitment and downstream transcriptional activity in ways distinct from natural hormones.
Steroidogenic Pathways: Many EDCs alter the expression and activity of enzymes involved in steroid hormone synthesis, including aromatase (CYP19), 5α-reductase, and various hydroxysteroid dehydrogenases [82]. This can lead to altered local and systemic hormone concentrations, particularly during sensitive developmental windows. For example, prenatal BPA exposure can reduce testosterone production in fetal testes by interfering with cholesterol transport and steroidogenic acute regulatory protein (StAR) expression [82].
Neurodevelopmental Pathways: EDCs disrupt signaling pathways critical for brain development, including those involving thyroid hormones, estrogens, and androgens [81]. These disruptions can alter neurogenesis, neuronal migration, apoptosis, and synaptogenesis, potentially contributing to the increased prevalence of neurodevelopmental and psychiatric disorders [81]. The hypothalamus, amygdala, prefrontal cortex, and bed nucleus of the stria terminalis (BNST) appear particularly vulnerable to EDC-induced alterations [84].
EDC Mechanism of Action
Integration of EDC Effects Across Biological Scales
The effects of EDCs integrate across multiple biological scales, from molecular interactions to organism-level phenotypes. At the molecular level, EDC interactions with receptors and epigenetic machinery alter gene expression patterns and cellular signaling. These molecular changes disrupt cellular processes including proliferation, differentiation, and apoptosis, leading to tissue-level alterations in organ development and function. Ultimately, these disruptions manifest as organism-level phenotypes including metabolic disorders, reproductive impairments, neurobehavioral abnormalities, and disease susceptibility [81] [83].
The non-monotonic dose-response relationships of EDCs further complicate this picture, as low doses may produce effects opposite to those observed at high doses. Additionally, the "cocktail effects" of mixture exposures may produce unexpected outcomes not predictable from single-chemical studies. Understanding these complex interactions requires integrated approaches that span multiple biological scales and utilize both traditional toxicological methods and novel systems biology approaches.
Research Toolkit: Essential Reagents and Methodologies
Core Reagents and Experimental Systems
Table 3: Essential Research Tools for EDC Studies
| Category | Specific Examples | Research Application |
|---|---|---|
| Reference EDCs | Bisphenol A (BPA), Di-(2-ethylhexyl) phthalate (DEHP), Vinclozolin | Positive controls for experimental studies |
| Cell-Based Models | MCF-7 cells (ER+), MDA-MB-231 cells (ER-), primary gonadal cells | Rapid screening of endocrine activity |
| Animal Models | CD-1 mice, Sprague-Dawley rats, zebrafish | In vivo assessment of developmental and transgenerational effects |
| Molecular Reagents | ERα/β antibodies, AR antibodies, DNA methylation kits | Mechanistic studies of endocrine disruption |
| Analytical Standards | Deuterated EDCs, certified reference materials | Quantification of exposure levels and metabolism |
Advanced Methodological Approaches
Contemporary EDC research utilizes sophisticated methodological approaches to address the complexities of low-dose and transgenerational effects:
Epigenomic Mapping: Techniques including whole-genome bisulfite sequencing, ChIP-seq for histone modifications, and ATAC-seq for chromatin accessibility enable comprehensive profiling of epigenetic changes induced by EDC exposure [82] [83]. These approaches are particularly valuable for identifying epigenetic signatures that may be transmitted across generations.
Multi-Omics Integration: Combining transcriptomic, proteomic, metabolomic, and epigenomic datasets provides systems-level understanding of EDC effects. Integrative analyses can reveal novel pathways and networks disrupted by EDCs and identify potential biomarkers of effect and exposure.
Computational Toxicology: In silico approaches including molecular docking simulations, quantitative structure-activity relationship (QSAR) modeling, and adverse outcome pathway (AOP) development help prioritize EDCs for further testing and predict potential health risks [85]. Mendelian randomization and colocalization analyses can provide insights into causal relationships between EDC exposure and health outcomes using human genomic data [85].
Novel Exposure Assessment: Beyond traditional biomonitoring in blood and urine, emerging approaches include hair analysis for retrospective exposure assessment, silicone wristbands for personal exposure monitoring, and wastewater-based epidemiology for population-level exposure estimation [13].
The study of low-dose effects and transgenerational impacts of EDCs represents a critical frontier in environmental health science. The evidence reviewed demonstrates that EDCs can produce significant biological effects at environmentally relevant exposure levels and that these effects may persist across multiple generations through epigenetic mechanisms. These findings challenge traditional toxicological paradigms and regulatory approaches that assume threshold doses for toxicity and focus primarily on high-dose effects.
Future research must address several critical knowledge gaps, including the mechanisms underlying non-monotonic dose responses, the complex interactions in EDC mixtures, the translation of epigenetic changes from germ cells to somatic phenotypes in subsequent generations, and the relevance of animal model findings to human health [28]. Additionally, there is an urgent need to develop regulatory frameworks that account for low-dose and transgenerational effects, particularly for protecting vulnerable populations during critical windows of development.
The continued development and application of innovative methodological approaches – including multi-generational study designs, advanced epigenomic technologies, and integrated data analysis strategies – will be essential for advancing our understanding of these complex phenomena. Ultimately, addressing these knowledge gaps will inform evidence-based policies to reduce EDC exposure and protect public health across current and future generations.
The paradigm of toxicological risk assessment is undergoing a fundamental shift, moving from the traditional evaluation of single chemicals toward a more realistic framework that addresses complex mixtures of environmental stressors. Cumulative risk assessment (CRA) represents a methodological approach for evaluating the combined risks from exposure to multiple chemical and non-chemical stressors that may contribute to a common adverse health outcome [86]. This approach is particularly critical for understanding the public health impact of endocrine-disrupting chemicals (EDCs), where humans are consistently exposed to complex mixtures through food, air, and skin absorption routes rather than to isolated compounds in laboratory settings [13] [70].
The scientific and regulatory impetus for this shift stems from the recognition that real-world exposures occur as complex combinations, and that these mixtures can produce health effects even when individual components are present at seemingly safe levels [87]. Research has demonstrated that EDCs, including pesticides, plastic additives, and per- and polyfluoroalkyl substances (PFAS), can disrupt hormonal balance and reproductive health across the lifespan, with effects manifesting from fetal development through adulthood [70]. The challenge lies in developing robust methodologies to accurately characterize these combined exposures and their integrated health impacts, particularly for sensitive endpoints such as reproductive function and development.
Table: Key Definitions in Cumulative Risk Assessment
| Term | Definition | Relevance to EDC Research |
|---|---|---|
| Cumulative Risk Assessment | Evaluation of combined risks from multiple stressors that may contribute to common adverse outcomes [86] | Framework for assessing real-world EDC mixture exposures |
| Dose Addition | Model where chemicals contribute proportionally to a common effect based on their individual potencies [86] [87] | Default assumption for EDCs affecting same adverse outcome through similar or different mechanisms |
| Common Mechanism Grouping | Traditional approach grouping chemicals that share a specific mechanism of toxicity [86] | Limited application for diverse EDCs with disparate molecular initiating events |
| Disease-Centered Grouping | Emerging approach grouping chemicals that contribute to a common disease or adverse outcome through different pathways [86] [87] | More protective framework for EDCs targeting reproductive health |
| Adverse Outcome Pathway (AOP) | Conceptual framework linking molecular initiating events to adverse outcomes through measurable key events [86] | Organizing structure for hypothesizing and testing EDC mixture effects |
Methodological Frameworks for Mixture Assessment
Component-Based Approaches
The predominant methodology in cumulative risk assessment involves component-based approaches that utilize data from individual chemicals and additivity models to estimate mixture effects [86] [87]. This approach depends on two critical assumptions: first, that chemicals will act jointly according to a specified model of additivity (typically dose addition); and second, that the mixture components will not interact in ways that produce significantly greater-than-additive (synergistic) or less-than-additive (antagonistic) effects [86].
Dose addition has emerged as a reasonable default assumption for chemicals within a common assessment group, where the combined effect equals the sum of the scaled doses of the individual components [87]. This model underpins several established risk assessment frameworks, including:
- Toxic equivalency factors applied to dioxin-like chemicals
- Relative potency factors for mixtures of polyfluoroalkyl substances [86]
- Cumulative risk assessments for pesticide classes such as organophosphates, N-methyl carbamates, and pyrethroids [86]
The critical scientific question becomes determining which chemicals belong in a common assessment group. While regulatory mandates have traditionally required grouping based on common mechanism of toxicity, this approach has proven resource-intensive and has resulted in only a limited number of established chemical classes with quantitative cumulative risk assessments [86].
Disease-Centered Grouping Using Adverse Outcome Pathways
An emerging alternative to mechanism-based grouping is the disease-centered approach, which organizes chemicals based on their contribution to a common disease or adverse outcome, regardless of their specific mechanisms of action [86] [87]. This framework leverages the Adverse Outcome Pathway concept as an organizing structure for developing and testing hypotheses about joint action [86].
The EuroMix project, funded through the European Union's Horizon 2020 program, has pioneered the application of AOP networks to evaluate mixtures of chemicals that impact specific adverse outcomes [86]. Their case study approach has demonstrated that dose addition provides a reasonable model for predicting mixture effects even for chemicals operating through different molecular initiating events that converge on common adverse outcomes.
Table: EuroMix Case Studies Demonstrating Disease-Centered Grouping
| Case Study | Chemicals Evaluated | Molecular Initiating Events | Key Finding |
|---|---|---|---|
| Liver Steatosis | Imazalil, thiacloprid, clothianidin, tebuconazole [86] | Activation of PPARα, PXR, AhR receptors [86] | Dose addition observed for triglyceride accumulation in vitro and in vivo |
| Craniofacial Malformations | Diverse chemicals with different mechanisms [86] | Multiple disparate molecular targets [86] | Binary mixtures conformed to dose additivity in zebrafish models |
| Male Reproductive Tract Disruption | Dienestrol (estrogenic), linuron and flutamide (antiandrogenic) [86] | Estrogen receptor activation, androgen receptor antagonism [86] | Flutamide drove observed effects; dose addition appropriate for mixtures with different mechanisms |
Experimental Protocols for Mixture Toxicology
In Vitro Testing Strategies
Comprehensive assessment of chemical mixtures requires a tiered testing approach that begins with high-throughput in vitro systems. The following protocol outlines a standardized methodology for evaluating mixture effects using human cell lines:
Protocol: In Vitro Assessment of EDC Mixtures for Reproductive Toxicity
Cell Model Selection:
- Utilize relevant cell lines such as human HepaRG cells for hepatic effects or H295R adrenal carcinoma cells for steroidogenesis assessment [86].
- Culture cells according to standard conditions appropriate for the selected cell line.
Test Chemical Preparation:
- Prepare individual stock solutions of each EDC in appropriate solvents (DMSO, ethanol) based on solubility.
- Create mixture ratios based on environmental relevance or equipotent proportions relative to individual points of departure.
Dosing and Exposure:
- Expose cells to individual chemicals and mixtures across a concentration range encompassing anticipated effect levels.
- Include appropriate vehicle controls and positive controls specific to the endpoint being measured.
- Maintain exposure for duration sufficient to observe key events (typically 24-72 hours for acute effects).
Endpoint Measurement:
- Assess cytotoxicity using standardized assays (MTT, Alamar Blue, ATP content).
- Measure specific key events using appropriate functional assays:
- Receptor activation using reporter gene assays
- Steroid hormone production using ELISA or LC-MS/MS
- Gene expression of key pathway markers using qRT-PCR
- Triglyceride accumulation for steatotic effects (Oil Red O staining) [86]
Data Analysis:
- Calculate individual chemical potency values (EC₅₀, BMC).
- Compare observed mixture responses to predictions generated by dose addition and independent action models.
- Use statistical approaches (ANOVA, regression) to identify deviations from additivity.
In Vivo Validation Studies
After initial in vitro characterization, targeted in vivo studies provide critical validation of mixture effects in whole organisms. The following protocol outlines a standardized approach for assessing reproductive effects of EDC mixtures:
Protocol: In Vivo Assessment of EDC Mixtures on Reproductive Development
Animal Model Selection:
- Use appropriate sensitive species and strains (e.g., Wistar rats, CD-1 mice) [86].
- Ensure adequate sample size per group (typically 10-12 animals) to achieve statistical power.
Exposure Paradigm:
- Time exposures to critical developmental windows (gestational, lactational, peripubertal).
- Administer mixtures via environmentally relevant routes (oral gavage, diet, drinking water).
- Include dose groups that reflect real-world exposure ratios and concentrations.
Endpoint Assessment:
- Organ weights: Reproductive organs (testes, ovaries, uterus), liver, kidneys, adrenals.
- Histopathology: Comprehensive evaluation of reproductive tissues.
- Serum hormone analysis: Testosterone, estradiol, LH, FSH, thyroid hormones.
- Gene and protein expression: Key markers in reproductive tissues and hormone-responsive organs.
Statistical Analysis:
- Use appropriate statistical models to detect additive and interactive effects.
- Apply benchmark dose modeling for potency estimation.
- Conduct pathway analysis on transcriptomic and proteomic data.
Data Analysis and Interpretation
Quantitative Approaches for Mixture Effects
The core analytical challenge in cumulative risk assessment lies in accurately predicting mixture effects from individual component data. The following quantitative approaches represent current best practices:
Dose Addition Modeling: The dose addition model assumes that chemicals in a mixture act as dilutions of one another, contributing to the overall effect in proportion to their individual potencies. The model is mathematically represented as:
[ \sum{i=1}^{n} \frac{di}{ED{xi}} = 1 ]
Where (di) is the dose of the i-th chemical in the mixture, and (ED{x_i}) is the dose of the i-th chemical that alone produces x% effect.
Statistical Testing for Deviations from Additivity: Rigorous statistical methods are required to distinguish additive from interactive effects. The following approaches are recommended:
- Response surface methodology for characterizing interaction profiles
- Two-stage linear regression for detecting significant deviations
- Benchmark dose modeling for estimating composite potency
Table: Data Requirements for Cumulative Risk Assessment of EDCs
| Data Type | Specific Elements | Source/Method |
|---|---|---|
| Exposure Data | Concentration in food, air, water, consumer products; biomonitoring data (blood, urine) [70] | NHANES, environmental monitoring, dietary surveys |
| Toxicity Data | Points of departure (BMD, NOAEL, LOAEL), potency estimates, mechanism/mode of action | ToxCast/Tox21, traditional toxicity testing, literature review |
| Physicochemical Properties | Persistence, bioaccumulation potential, half-lives | Laboratory testing, QSAR models |
| Population Susceptibility | Life stage vulnerabilities, genetic polymorphisms, pre-existing conditions | Epidemiological studies, biomarker research |
| Temporal Factors | Exposure timing, critical windows, cumulative body burden | Longitudinal studies, cohort analyses |
Research Reagent Solutions for EDC Mixture Studies
Table: Essential Research Materials for EDC Mixture Toxicology
| Reagent/Category | Specific Examples | Research Application |
|---|---|---|
| Reference EDCs | Vinclozolin, flutamide, diethylstilbestrol, BPA, PFOS, phthalates [86] [70] | Positive controls for mechanism-specific screening; potency calibration |
| Cell-Based Bioassay Systems | MVLN cells (ER activation), AR-EcoScreen cells (AR antagonism), H295R cells (steroidogenesis) [86] | High-throughput screening of receptor-mediated effects and hormone production |
| Analytical Standards | Isotope-labeled EDCs, metabolite standards, internal standards for LC-MS/MS | Quality control for exposure assessment; biomarker quantification |
| AOP-Specific Antibodies | Phospho-specific antibodies for signaling proteins; receptors (ERα, AR, AhR); steroidogenic enzymes (CYP19A1, CYP17A1) [86] | Pathway perturbation assessment in in vitro and in vivo systems |
| qPCR Assays | AOP-relevant gene panels (steroid receptors, hormone synthesizing enzymes, developmental regulators) [86] | Key event measurement in tiered testing strategies |
Visualization of Cumulative Risk Assessment Framework
Cumulative Risk Assessment Framework
Experimental Workflow for Mixture Testing
Mixture Testing Workflow
The assessment of complex chemical mixtures represents one of the most significant challenges in modern toxicology and environmental health. The framework outlined in this review provides a scientifically robust approach for addressing the cumulative risks posed by EDCs and other environmental stressors. The transition from mechanism-based to disease-centered grouping, supported by AOP networks and dose addition modeling, offers a more protective and biologically plausible foundation for cumulative risk assessment [86] [87].
Future advancements in this field will require continued development of innovative testing strategies, refined quantitative models, and integration of novel data streams from high-throughput screening and computational toxicology. Additionally, there is growing recognition of the need to incorporate non-chemical stressors into cumulative risk frameworks, as factors such as psychosocial stress and socioeconomic status may modify susceptibility to chemical exposures [86]. As evidence continues to accumulate regarding the health impacts of real-world mixture exposures, the scientific and regulatory communities must embrace these advanced methodologies to adequately protect public health, particularly for vulnerable populations and sensitive life stages [70].
Strategies for Exposure Reduction in Vulnerable Populations
Endocrine-disrupting chemicals (EDCs) are natural or human-made chemicals that can mimic, block, or interfere with the body's hormones, which are part of the endocrine system [2]. These chemicals are linked with a wide array of health issues and are found in many everyday products, including cosmetics, food and beverage packaging, toys, carpet, and pesticides [2]. Exposure to these chemicals occurs primarily through dietary intake, inhalation, and dermal absorption [88], making them a pervasive public health challenge.
Vulnerable populations, particularly pregnant women, fetuses, infants, and children, face heightened risks from EDC exposures. The fetus, infant, and child may have enhanced sensitivity to environmental stressors like EDCs due to rapid development and greater exposure resulting from developmentally appropriate behavior, anatomy, and physiology [43]. For instance, infants and children may have higher exposure to some EDCs than adults because they consume more water and greater quantities of specific foods, have higher ventilation rates, intestinal absorption, surface area to volume ratios, and engage in frequent hand-to-mouth activity [43]. Additionally, many EDCs can cross the placental barrier, exposing the fetus directly during critical developmental windows [35].
Critical Exposure Pathways and Mechanisms
Dietary Exposure Pathways
Diet represents one of the most significant and consistent pathways of EDC exposure [35]. The food supply has been shown to contain EDCs, including pesticide residues, phthalates, bisphenols, and persistent organic pollutants (POPs) [35].
- Bisphenols: Used in the production of epoxy resins and polycarbonate plastics frequently found in food containers, beverage bottles, and the linings of canned goods [35]. These substances can infiltrate food and drink, particularly in hot or acidic environments [35]. Bisphenol A (BPA) and its substitutes (BPS and BPF) have been detected in maternal urine and amniotic fluid, indicating direct exposure to the developing fetus [35].
- Phthalates: Used as plasticizers to make plastics more flexible in processing materials, storage equipment, and food packaging [35]. These substances can readily seep into foods, especially those high in fat, such as meat, dairy products, and oils, because they are not chemically bonded to plastics [35].
- Persistent Organic Pollutants (POPs): Compounds like dioxins and polychlorinated biphenyls (PCBs) bioaccumulate in the food chain and are frequently consumed through meat, fish, and dairy products [35].
Table 1: Major Dietary EDCs and Their Primary Food Sources
| EDC Class | Specific Chemicals | Common Food Sources |
|---|---|---|
| Bisphenols | BPA, BPS, BPF | Canned foods and beverages, polycarbonate plastic containers, thermal receipts [35] |
| Phthalates | DEHP, DnBP, DiBP, DEP | Fatty foods (meat, dairy, oils), food packaging, plastic-wrapped foods [35] |
| Persistent Organic Pollutants | Dioxins, PCBs | Meat, fish, dairy products (via bioaccumulation) [35] |
| Pesticides | Organophosphates, Organochlorines | Fruits, vegetables, grains [35] |
Inhalation and Dermal Exposure Pathways
Beyond dietary exposure, EDCs enter the body through inhalation of chemicals and dermal contact [88]. Phthalates, for example, are used in hundreds of products including cosmetics, fragrances, children's toys, and medical device tubing [2]. Cosmetics that may contain phthalates include nail polish, hair spray, aftershave lotion, cleanser, and shampoo [2]. These compounds can be directly absorbed through the skin or inhaled when volatilized into the air.
The lipophilic nature of many EDCs enables them to easily pass through biological barriers including skin and placenta [35]. This property facilitates accumulation in fatty tissues and contributes to their persistence in the body and environment. Dermal exposure occurs through direct contact with personal care products, household dust, and contaminated surfaces, while inhalation exposure predominantly results from airborne EDCs in indoor environments where these chemicals leach from products and building materials.
Quantified Health Risks and Exposure Data
Epidemiological studies have quantified the relationship between EDC exposure and adverse health outcomes, particularly in vulnerable populations. The timing of exposure is critical, with prenatal and early childhood periods representing windows of heightened susceptibility.
Table 2: Quantified Health Risks from Prenatal EDC Exposure
| EDC Class | Health Outcome | Risk Quantification | Study Population |
|---|---|---|---|
| Bisphenol A (BPA) | Lower birth weight | Top quartile of maternal urinary BPA associated with 1.4-fold greater odds of lower birth weight (95% CI: 1.1-1.9) [35] | Multiple cohorts |
| Bisphenol A (BPA) | Neurobehavioral changes | Top quartile of maternal urinary BPA associated with 1.6-fold higher chances of neurobehavioral changes (95% CI: 1.1-1.9) [35] | Multiple cohorts |
| Di(2-ethylhexyl)phthalate (DEHP) | Impaired male reproductive development | Highest quartile of DEHP metabolites associated with 1.9-times greater likelihood of impaired genital development (OR = 1.87; 95% CI: 1.12-3.12) [35] | Cohort studies |
| Di(2-ethylhexyl)phthalate (DEHP) | Childhood wheeze | Highest quartile of DEHP metabolites associated with twice the likelihood of wheeze (OR = 2.03; 95% CI: 1.15-3.57) [35] | Cohort studies |
| Phthalates (general) | Preterm birth | Exposure to certain phthalates associated with decreased gestational age and increased risk of preterm birth [2] | Diverse U.S. birth sample |
Research indicates that EDCs may increase the risk of childhood diseases by disrupting hormonally mediated processes critical for growth and development during gestation, infancy, or childhood [43]. The available epidemiological evidence suggests that prenatal exposure to several ubiquitous EDCs is associated with adverse neurobehavior (BPA and phthalates) and excess adiposity or increased risk of obesity/overweight (PFAS) [43].
EDC Exposure Pathways and Physiological Distribution
The following diagram illustrates the primary exposure routes and internal distribution of EDCs within vulnerable populations:
This pathway visualization illustrates how EDCs enter the body through three primary exposure routes (dietary, inhalation, and dermal), undergo absorption and distribution through various physiological processes, and ultimately reach vulnerable target systems, with particular concern for placental and lactational transfer to developing offspring.
Evidence-Based Exposure Reduction Strategies
Dietary Intervention Strategies
Dietary modifications represent the most effective approach for reducing EDC exposure in vulnerable populations. Research demonstrates that targeted dietary interventions can significantly reduce body burden of EDCs.
- Fresh Food Substitution: Implementing a fresh food diet with limited packaged and processed foods significantly reduces exposure to bisphenols and phthalates. A dietary intervention study replacing canned foods and food packaged in plastic with fresh alternatives demonstrated reductions in urinary BPA (66%) and DEHP metabolites (53-56%) within just three days [89].
- Food Storage Modifications: Avoid storing canned or plastic-packaged foods in hot environments (e.g., car trunks), and refrain from microwaving food in plastic containers, as heat promotes leaching of EDCs from packaging into food [90].
- Water Filtration: Use filtered tap water instead of bottled water to reduce phthalate exposure, as bottled water may contain plasticizers that leach into the water [90].
- Produce Washing: Wash fresh fruits and vegetables with tap water to remove most surface pesticide residues and reduce dietary exposure to agricultural chemicals [90].
Consumer Product Selection Strategies
Informed consumer choices can substantially reduce EDC exposure from non-dietary sources:
- Plastic Product Identification: On plastic bottles, a #1, #2, or #4 in the recycling symbol indicates the product is free of BPA [90]. Similarly, PVC-free labels on shower curtains, raincoats, flooring, and furniture reduce phthalate exposure.
- Personal Care Product Selection: Read labels for cleaning supplies, facial washes, and detergents that indicate the presence or absence of known EDCs like phthalates [90]. Select products specifically labeled as phthalate-free.
- BPA-Free Alternatives: Choose canned foods with BPA-free liners and seek merchants that disclose EDC status for their products [90]. Some grocery stores now list yes/no status for certain EDCs in their products.
Household-Level Exposure Control
- Pesticide Use Reduction: Implement integrated pest management strategies like plugging holes and removing food sources to reduce the need for chemical pesticides [90].
- Dust Control: Regular wet mopping and vacuuming with HEPA filters reduces household dust containing phthalates, flame retardants, and other semi-volatile EDCs that settle from indoor air.
- Ventilation Improvement: Increased ventilation reduces concentrations of airborne EDCs that accumulate in indoor environments from off-gassing of building materials, furniture, and household products.
Experimental Protocols for Exposure Assessment
Biomonitoring Methods for EDC Exposure Assessment
Accurate assessment of EDC exposure is essential for both research and monitoring the effectiveness of exposure reduction interventions. The following protocol outlines standardized approaches for EDC biomonitoring:
Protocol 1: Urinary Biomarker Assessment for Non-Persistent EDCs
Application: This protocol is optimized for measuring non-persistent EDCs including bisphenols, phthalates, parabens, and triclosan with short biological half-lives (6 hours to 3 days) [89].
Sample Collection:
- Collect first-morning void urine samples in chemically-screened containers to avoid contamination.
- Process samples by aliquoting and freezing at -80°C within 4 hours of collection to prevent degradation.
- For pregnant women, collect serial samples across trimesters to capture exposure variability during critical developmental windows.
Chemical Analysis:
- Utilize liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) for precise quantification of parent compounds and metabolites.
- Analyze specific phthalate metabolites including monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), mono-isobutyl phthalate (MiBP), and mono-(2-ethylhexyl) phthalate (MEHP).
- Quantify bisphenol A (BPA) and its substitutes (BPS, BPF) alongside paraben biomarkers (methylparaben, propylparaben, butylparaben).
Quality Assurance:
- Implement isotope dilution methods using (^{13}\text{C})-labeled internal standards for each analyte to correct for matrix effects and recovery variations.
- Include method blanks, quality control pools, and standard reference materials (when available) with each analytical batch.
- Adjust for urinary dilution using specific gravity or creatinine measurements.
Protocol 2: Serum Assessment for Persistent EDCs
Application: This protocol measures persistent EDCs including perfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), and organochlorine pesticides that accumulate in biological tissues.
Sample Collection:
- Collect fasting blood samples in serum separator tubes.
- Allow samples to clot for 30 minutes at room temperature before centrifugation.
- Store aliquoted serum at -80°C until analysis.
Chemical Analysis:
- Utilize solid phase extraction followed by LC-MS/MS for PFAS analysis.
- Employ gas chromatography with high-resolution mass spectrometry (GC-HRMS) for PCB and organochlorine pesticide quantification.
- Quantify major PFAS including perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorohexanesulfonic acid (PFHxS), and perfluorononanoic acid (PFNA).
Intervention Efficacy Assessment Protocol
Protocol 3: Evaluating Exposure Reduction Interventions
Application: This protocol provides a framework for assessing the effectiveness of exposure reduction strategies in vulnerable populations.
Pre-Intervention Assessment:
- Administer environmental health literacy (EHL) surveys assessing knowledge of EDC sources and exposure pathways.
- Measure readiness to change (RtC) using validated scales to assess motivation for adopting exposure-reduction behaviors.
- Collect baseline biospecimens (urine, serum) for EDC biomarker analysis.
Intervention Implementation:
- Implement multi-component interventions including:
- Personalized report-back of biomarker results with source identification
- Educational curricula on EDC exposure pathways and reduction strategies
- Environmental assessments identifying exposure sources in home environments
- Provision of replacement products (e.g., glass food containers, alternative personal care products)
- Implement multi-component interventions including:
Post-Intervention Evaluation:
- Repeat EHL and RtC assessments to measure changes in knowledge and motivation.
- Collect follow-up biospecimens 2-3 months post-intervention to measure changes in EDC biomarkers.
- Administer behavioral surveys to assess adoption of exposure-reduction practices.
Statistical Analysis:
- Use paired t-tests or Wilcoxon signed-rank tests to compare pre- and post-intervention biomarker concentrations.
- Employ multiple regression models to identify predictors of successful exposure reduction.
- Calculate percentage reduction in urinary metabolite concentrations using geometric means to account with right-skewed distributions.
Research Reagent Solutions for EDC Studies
Table 3: Essential Research Reagents for EDC Exposure and Intervention Studies
| Reagent/Chemical | Application in EDC Research | Example Use Case |
|---|---|---|
| Certified Reference Standards (Native and isotope-labeled) | Quantification of EDCs and metabolites in biological matrices | Internal standards for LC-MS/MS analysis of urinary bisphenols and phthalate metabolites [89] |
| Quality Control Materials | Method validation and quality assurance | Commercially available pooled urine/serum with certified analyte concentrations for batch-to-batch QC |
| Solid Phase Extraction (SPE) Cartridges | Sample clean-up and pre-concentration | Extraction of EDCs from urine/serum prior to LC-MS/MS analysis |
| LC-MS/MS Grade Solvents | Mobile phase preparation and sample extraction | High purity solvents (methanol, acetonitrile, water) to minimize background contamination |
| Biomarker Assay Kits | Assessment of clinical health biomarkers | Commercially available tests for hormones, metabolic markers, and inflammatory cytokines [89] |
| Standard Reference Materials (NIST) | Method validation and accuracy assessment | Certified reference materials with assigned values for EDCs in human urine/serum |
Intervention Testing and Implementation Framework
The following diagram outlines a comprehensive framework for developing and testing EDC exposure reduction interventions:
This framework illustrates the systematic approach to EDC exposure reduction, beginning with comprehensive baseline assessment, implementing multi-component interventions, and measuring outcomes across exposure, knowledge/behavior, and clinical biomarkers.
Effective strategies for reducing EDC exposure in vulnerable populations require multi-faceted approaches addressing dietary, inhalation, and dermal exposure pathways. Evidence demonstrates that interventions incorporating biomonitoring with personalized report-back, targeted educational curricula, and practical exposure reduction strategies can significantly reduce body burdens of EDCs, even those with short biological half-lives [89].
Future research directions should prioritize:
- Mixture Effects Assessment: Moving beyond single-chemical studies to evaluate the cumulative effects of real-world EDC mixtures [43].
- Sensitive Window Identification: Elucidating periods of heightened vulnerability across the lifespan to prioritize intervention timing [43].
- Clinical Biomarker Integration: Linking EDC exposure reduction to improvements in validated health biomarkers to demonstrate clinical relevance [89].
- Regulatory Policy Evaluation: Assessing the impact of policy interventions on population-level EDC exposures and health outcomes [17].
The implementation of evidence-based exposure reduction strategies, coupled with ongoing research to address critical knowledge gaps, represents a promising approach to protecting vulnerable populations from the adverse health effects associated with EDC exposure.
Clinical trials are undergoing a significant transformation, driven by the need for greater efficiency, enhanced data quality, and robust patient safety protections. The traditional approach of extensive on-site monitoring and 100% Source Data Verification (SDV) is increasingly being replaced by more sophisticated, proactive strategies. Risk-Based Monitoring (RBM) and advanced Data Quality Management form the cornerstone of this modernized approach, enabling sponsors to direct resources and attention to the most critical trial aspects. These methodologies are particularly vital within complex research fields like the study of endocrine-disrupting chemicals (EDCs), where precise exposure assessment and high-integrity data are paramount for understanding health impacts via food, air, and skin absorption routes.
The integration of these approaches allows for a more holistic view of clinical trial data, facilitating earlier identification of emergent issues and enabling data-driven decisions more quickly [91]. This is essential in EDC research, where data must not only be clinically sound but also accurately capture environmental exposure metrics. This guide provides a technical deep dive into the core principles, methodologies, and practical applications of RBM and data quality management, framed for researchers and drug development professionals operating in this demanding field.
Foundations of Risk-Based Monitoring (RBM)
Risk-Based Monitoring (RBM) is an adaptive, proactive method of clinical trial monitoring that directs monitoring focus and activities to the evolving areas of greatest need which have the most potential to impact patient safety and data quality [92]. It represents a fundamental shift from reactive, one-size-fits-all oversight to a targeted, strategic model.
Core Principles and Benefits of RBM
The philosophy of RBM is built on the systematic application of quality-by-design and risk management principles throughout the trial lifecycle. The primary objective is to ensure the protection of trial participants and the reliability of study results, while optimizing the use of resources. A key manifestation of this approach is Centralized Monitoring, which "provides a holistic view of aggregate clinical trial data and earlier identification of emergent issues, enabling organizations to make data-driven decisions more quickly" [91]. This is a marked departure from relying solely on periodic site visits.
The benefits of a well-executed RBM strategy are substantial:
- Enhanced Patient Safety: Continuous, centralized oversight allows for faster detection of potential safety signals.
- Improved Data Quality: Focusing efforts on critical data points and processes reduces errors where it matters most.
- Increased Operational Efficiency: By reducing unnecessary on-site visits and redundant SDV, RBM can significantly lower trial costs and alleviate site burden.
- Proactive Risk Management: The model facilitates the early identification of issues at specific sites or across the study, allowing for mitigation before problems escalate.
The RBM Workflow: A Strategic Process
Implementing RBM is a continuous cycle, not a one-time event. The workflow can be visualized as a series of interconnected stages, from initial planning to operational execution and continuous refinement. The following diagram illustrates this iterative process and the key activities at each stage.
Figure 1: The iterative, six-stage Risk-Based Monitoring workflow, from initial planning to continuous refinement.
As outlined in Figure 1, the RBM process begins with the identification of critical data and processes—those elements most essential to patient safety and trial outcomes. For EDC research, this explicitly includes exposure assessment methodologies for food, air, and skin routes. The subsequent risk assessment evaluates the likelihood and impact of failures. Mitigation strategies are then designed, heavily leveraging centralized monitoring techniques and the tracking of Key Risk Indicators (KRIs). This proactive planning informs the ongoing monitoring activities during trial execution, where data is continuously analyzed to trigger corrective actions, leading to a refined and adapted monitoring strategy.
A Framework for Clinical Data Quality Management
High-quality data is the lifeblood of any successful clinical trial. It is defined as data that is fit for purpose, accurate, complete, consistent, and reliable [93]. In the context of EDC research, where the goal is often to detect subtle effects from low-level environmental exposures, uncompromising data quality is non-negotiable. Poor data quality costs organizations an average of $12.9 million annually and leads to missed opportunities for competitive advantage [93] [94].
The Dimensions of Data Quality
Data quality is measured across multiple dimensions, each representing a different aspect of data fitness. The table below summarizes the eight fundamental dimensions critical for clinical research, especially pertinent to the complex data types encountered in EDC studies.
Table 1: Key Dimensions of Data Quality in Clinical Research
| Dimension | Description | Relevance to EDC Research |
|---|---|---|
| Completeness | Absence of missing records, gaps, or undefined values. | Essential for longitudinal exposure tracking and biomarker data. |
| Accuracy | Data reflects the real-world without errors. | Critical for analytical chemistry results (e.g., EDC concentration levels). |
| Consistency | Same data is represented similarly across different systems. | Ensures harmonization between clinical data and environmental data. |
| Timeliness | Data is current and available when needed. | Vital for safety monitoring and adaptive trial designs. |
| Uniqueness | Absence of duplicate records. | Prevents over-counting of participant exposures or events. |
| Validity | Data complies with predetermined rules, formats, and value ranges. | Ensures coded EDC exposures and lab values fall within expected ranges. |
| Accessibility | Data is easily accessible to authorized users. | Supports collaborative research across multiple institutions. |
| Relevance | Data is directly related to the user and purpose of use. | Ensures collected exposure variables align with research endpoints. |
The Data Quality Management Lifecycle
Managing data quality is not a one-time project but a continuous commitment [94]. It requires a systematic, multi-step approach that shifts from reactive cleanup to proactive prevention. The lifecycle is a closed loop, ensuring continuous improvement and adaptation to new challenges, such as those presented by mixed EDC exposures.
Figure 2: The continuous Data Quality Management lifecycle, from governance to monitoring and back.
As shown in Figure 2, the cycle begins with Govern & Plan, establishing the strategic framework, data governance council, and data quality plan with clear, measurable objectives [94]. The next phase, Profile & Analyze, involves using automated tools to scan data sources to discover the frequency of null values, identify patterns, and uncover data quality issues, thus providing a baseline [94]. The Cleanse & Standardize step corrects errors in existing data, including rectifying inaccuracies, removing duplicates, and transforming data into a consistent format (e.g., ensuring all EDC units of measure are standardized) [94]. The Validate & Prevent phase is critical; it implements validation rules at the point of data entry to catch errors before they enter the system, which is highly effective for ensuring the validity of EDC exposure data collected via electronic data capture (EDC) systems [94]. Finally, the Monitor & Measure phase involves the ongoing tracking of data against defined quality metrics and KPIs, using dashboards and automated alerts to detect issues in real-time [94].
Practical Implementation: Protocols and Tools
Centralized Monitoring Protocol
Centralized monitoring is a cornerstone of RBM, utilizing statistical and analytical methods to review data accumulated from participating sites. The following is a detailed methodology for its implementation.
Table 2: Protocol for Implementing Centralized Monitoring in a Clinical Trial
| Step | Action | Description & Tools |
|---|---|---|
| 1. Define KRIs | Identify metrics signaling potential issues. | Examples: high screen-failure rates, unusual data distributions for key biomarkers, frequent protocol deviations related to exposure assessment. |
| 2. Configure Systems | Set up central monitoring platform. | Use platforms that facilitate setting up KRIs, identifying risks, and implementing mitigation actions [91]. |
| 3. Establish Baselines | Determine expected/normal ranges for KRIs. | Use historical trial data or preliminary data from the current trial. |
| 4. Continuous Review | Monitor aggregate data and KRI triggers. | Conduct regular reviews by a centralized monitoring team. |
| 5. Investigate & Act | Determine root cause of triggers. | Differentiate between systemic issues, site-specific problems, or data errors. |
| 6. Document & Report | Maintain audit trail of all activities. | Document all triggers, investigations, findings, and corrective actions. |
Data Validation and Cleansing Protocol
Ensuring data validity and accuracy requires a rigorous, multi-layered approach, particularly for the complex data types in EDC research.
Define Validation Rules: Before data collection begins, establish explicit rules for data formats, value ranges, and logical consistency. For example:
- Format Rule: Patient identifiers must follow a specific alphanumeric pattern (e.g.,
SiteID-P0001). - Range Rule: EDC biomarker concentration values must be within the quantifiable limits of the assay.
- Logic Rule: A date of a reported adverse event cannot precede the date of first exposure.
- Format Rule: Patient identifiers must follow a specific alphanumeric pattern (e.g.,
Implement at Point of Entry: Integrate these validation rules directly into the Electronic Data Capture (EDC) system. The system should perform automated checks and prevent or flag out-of-range or illogical entries in real-time [94]. For instance, if a user attempts to enter a phthalate level above the assay's upper limit of quantification, the EDC system would generate a query.
Conduct Post-Hoc Cleansing: After data collection, run systematic data profiling to identify errors that bypassed initial validation. This includes:
- Statistical Profiling: Identifying outliers in numerical data (e.g., an implausibly high value for a urinary EDC metabolite).
- Pattern Matching: Checking text fields for inconsistent entries (e.g., "canned food" vs. "Canned Food" in dietary logs).
- Cross-Record Checks: Ensuring consistency across related data points (e.g., confirming that a participant with high reported consumption of canned food also has detectable levels of BPA).
The Scientist's Toolkit: Essential Research Reagent Solutions
Success in EDC research and clinical trial management depends on a suite of specialized tools and platforms. This toolkit encompasses solutions for data capture, quality management, and exposure assessment.
Table 3: Essential Tools and Platforms for Modern Clinical Trials and EDC Research
| Tool Category | Example Solutions | Function & Application |
|---|---|---|
| Electronic Data Capture (EDC) | REDCap, Medidata RAVE, Oracle Clinical, Di-EDC [95] | Securely captures and manages clinical trial data; provides audit trails, real-time data access, and regulatory compliance. |
| Risk-Based Monitoring (RBM) | Centralized Monitoring Platforms [91] | Provides holistic, aggregate view of trial data; enables early issue identification and data-driven oversight. |
| Data Quality Management | Augmented Data Quality (ADQ) Solutions [93] | Uses AI/ML for automatic data profiling, anomaly detection, and rule discovery; often features natural language interfaces. |
| EDC Exposure Assessment | Validated Survey Questionnaires [60], Biomonitoring (blood, urine) [70], Wearable Sensors | Measures exposure to endocrine-disrupting chemicals via food, air, and skin; critical for defining study cohorts. |
| Data Governance & MDM | Master Data Management (MDM) Systems [94] | Creates a "single source of truth" for critical data (e.g., patient identifiers, product data), ensuring enterprise-wide consistency. |
The integration of Risk-Based Monitoring and strategic Data Quality Management represents a paradigm shift in how clinical trials are conducted. By focusing resources on critical risks and embedding quality into every stage of the data lifecycle, researchers can enhance patient safety, improve data integrity, and increase operational efficiency. For scientists investigating the subtle and complex effects of endocrine-disrupting chemicals, these modernized approaches are not merely an option but a necessity. They provide the rigorous framework required to generate reliable data on exposure routes and health outcomes, ultimately strengthening the scientific evidence base and informing public health policy. As the field evolves, the adoption of these principles, supported by emerging technologies like AI and augmented data quality solutions, will be the hallmark of successful and impactful clinical research.
Evaluating Evidence and Regulatory Frameworks: A Global Perspective on EDC Management
The Weight of Evidence (WoE) is a systematic, integrative approach used in scientific evaluation to assess the totality of available data related to a specific question. In the context of endocrine-disrupting chemical (EDC) safety and risk assessment, WoE methodology considers multiple lines of evidence to arrive at a balanced, scientifically justified conclusion rather than relying on a single study or data source [96]. This approach is particularly crucial for understanding EDC exposure through major routes including food, respiratory pathways, and skin absorption, where data may appear complex, variable, and occasionally contradictory [96] [13].
The WoE process involves synthesizing results from various study types, including human epidemiological studies, in vivo animal models, in vitro experiments, and computational models. Experts across multiple disciplines—toxicologists, chemists, immunologists—then critically appraise each piece of evidence for its quality, relevance, reliability, and consistency [96]. This methodology is essential for resolving inconsistencies between different data types; for instance, when a single in vitro study suggests potential harm at the cellular level, while in vivo animal studies show no adverse effects at relevant exposure levels, and epidemiological data indicates no observable impact on human populations [96].
The Weight of Evidence Framework: A Structured Methodology
Core Principles and Systematic Process
The WoE framework follows a structured process to weigh the strength, relevance, and reliability of data pertaining to EDC exposure routes. This systematic approach ensures robust decision-making by accounting for both the strengths and limitations of individual studies while identifying converging patterns or discrepancies across the broader body of research [96]. The methodology can be conceptualized as a multi-stage workflow:
Figure 1: Weight of Evidence Assessment Workflow
Data Collection: Comprehensive Evidence Gathering
The initial phase involves collecting all relevant data from diverse sources to ensure a comprehensive evidence base [96]:
Human Studies: Including clinical trials and observational research (e.g., cohort studies, case-control studies) that examine how EDCs affect human health under natural or controlled conditions. The NHANES dataset, for example, has been instrumental in providing population-level data on EDC exposure and health outcomes [15].
Animal Testing: Using living organisms such as mice or rats to observe how EDCs behave in whole-body systems over time, providing insights into potential mechanisms and dose-response relationships that cannot be ethically studied in humans [96].
In Vitro Studies: Laboratory-based experiments using cells or tissues to examine how EDCs affect biological processes in controlled environments. These include assays such as the ERα-CALUX system for detecting estrogenic activity [97].
New Approach Methodologies (NAMs): Advanced tools like "organs on a chip" that simulate human physiological responses without relying on animal models [96] [98].
Environmental Exposure Data: Information tracking how people encounter EDCs in air, water, food, or consumer products, and at what concentration levels [96].
Historical or Industry Safety Records: Long-term data from past use that helps identify patterns, rare effects, or well-established safety thresholds [96].
EDC Exposure Routes: Integrating Epidemiological and Toxicological Data
Primary Exposure Pathways and Their Methodological Considerations
EDCs enter the human body through three primary exposure routes: food, respiratory pathways, and skin absorption [13]. Each route presents distinct challenges and considerations for both epidemiological and toxicological research methodologies.
Table 1: EDC Exposure Routes: Methodological Approaches and Evidence Integration
| Exposure Route | Epidemiological Approaches | Toxicological Approaches | Key EDCs Studied | Evidence Integration Challenges |
|---|---|---|---|---|
| Food Ingestion | Dietary surveys, biomarker measurements in blood/urine, cohort studies linking exposure to health outcomes | In vitro digestion models, animal feeding studies, metabolic assays | Bisphenols, Phthalates, PFAS, Pesticides [13] [70] | Accounting for bioavailability, metabolic transformation, and mixed exposures |
| Respiratory Pathways | Air monitoring, indoor dust analysis, respiratory health questionnaires, lung function tests [12] | Inhalation studies in animals, lung cell culture models, particle deposition studies | Phthalates, Bisphenols, Flame retardants, Metals (Ni, Zn) [12] | Accounting for particle size, deposition patterns, and mucociliary clearance |
| Dermal Absorption | Personal care product use surveys, skin wipe sampling, biomonitoring | ex vivo skin models, permeation assays, 3D skin models | Parabens, Triclosan, Phthalates, UV filters [13] | Variations in skin permeability, vehicle effects, and limited systemic exposure data |
Quantitative Data Synthesis: Epidemiological Findings on EDC Health Effects
Epidemiological studies provide critical evidence linking EDC exposure to adverse health outcomes across different exposure routes. The table below synthesizes key quantitative findings from recent research:
Table 2: Epidemiological Evidence: EDC Exposure and Health Outcome Associations
| Health Outcome | EDC Class | Specific Compound(s) | Exposure Route | Effect Measure (OR, HR, RR) | Population | Source |
|---|---|---|---|---|---|---|
| Asthma Onset | Phthalates | DEHP, DiBP | Dust inhalation [12] | OR: 1.89 (1.00-3.57); OR: 1.41 (1.08-1.82) | Children & Adolescents | [12] |
| Asthma Onset | Bisphenols | BPA, BPS | Multiple routes [12] | OR: 1.57 (1.02-2.40); OR: 1.40 (1.13-1.73) | Children & Adolescents | [12] |
| Asthma Onset | Organophosphate esters | TBOEP | Dust inhalation [12] | OR: 2.61 (1.08-6.30) | Children & Adolescents | [12] |
| Asthma Exacerbation | Phthalate metabolites | MEHHP, MEOHP, MECPP | Multiple routes [12] | OR: 1.24 (1.02-1.51); OR: 1.30 (1.09-1.55); OR: 1.30 (1.07-1.57) | Children & Adolescents | [12] |
| Early Menopause | Pesticides & Phthalates | Multiple | Mixed [70] | Menopause 1.9-3.8 years earlier | Adult Women | [70] |
| PRISm (Pre-COPD) | Phthalates | MIBP | Multiple routes [15] | OR: 2.29 (1.71-3.07) | Adults | [15] |
Methodological Protocols: Experimental Approaches for EDC Research
For complex EDC evaluations where evidence is heterogeneous or incomplete, structured approaches like Expert Knowledge Elicitation (EKE) can be employed. This methodology captures expert judgment quantitatively while estimating uncertainty in the final opinion [99]. The protocol implemented for triphenyl phosphate (TPP) assessment demonstrates this approach:
Objective: Categorize the metabolic disruption properties of TPP through systematic literature review and expert evaluation [99].
Methodology:
- Conduct systematic literature review focusing on adverse endpoints (obesity/adipogenicity) and putative Molecular Initiating Events (activation of PPARγ)
- Assemble two independent groups of experts with diverse expertise
- Each group assesses quality of evidence lines separately using predefined criteria
- Reach categorical designation based on WoE
- Measure inter-group agreement to assess reproducibility [99]
Outcome: Both expert groups designated TPP as a "suspected metabolism-disrupting chemical" with quantitative agreement exceeding 85%, indicating robust reproducibility [99].
Translational Toxicology: Bridging In Vitro and In Vivo Findings
A significant challenge in EDC research is translating findings between experimental systems. A novel approach focuses on intracellular free concentration as a bridging metric [97]:
Experimental Protocol:
- Cell-Free Assay: Measure ERα activity using purified human estrogen receptor (hER)
- Cell-Based Assay: Quantify ERα activity using ERα-CALUX cells
- Uptake Kinetics: Determine cellular toxicokinetic parameters including cellular uptake and intracellular binding
- Computational Modeling: Predict free intracellular concentrations using in silico methods
- Correlation Analysis: Compare potency values between systems with and without toxicokinetic corrections [97]
Key Finding: Incorporating experimental toxicokinetic parameters significantly improved correlation between ERα activities in cell-free and cellular models (from r = 0.6230, P = 0.0989 without corrections to r = 0.8869, P = 0.0033 after corrections) [97].
Mixture Analysis Methods for Complex EDC Exposures
Humans are typically exposed to complex mixtures of EDCs, yet traditional risk assessment often focuses on single chemicals. Advanced statistical methods have been developed to address this limitation:
Weighted Quantile Sum (WQS) Regression:
- Creates a weighted index of mixed exposures
- Identifies "bad actor" chemicals driving overall effects
- Applied in PRISm study showing each quantile increase in EDC mixture associated with 63% higher odds of PRISm (OR=1.63, 95% CI: 1.25-2.13) [15]
Quantile g-Computation (Qgcomp):
- Estimates effect of simultaneous increase in all mixture components
- In PRISm analysis, showed 41% increased odds per index rise (OR=1.41, 95% CI: 1.15-1.72) [15]
Bayesian Kernel Machine Regression (BKMR):
- Flexible approach to capture complex exposure-response relationships
- Allows for non-linear and non-additive effects
- Confirmed positive overall mixture effect direction for EDCs and PRISm [15]
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents and Materials for EDC Investigation
| Reagent/Material | Application | Function | Example Use |
|---|---|---|---|
| ERα-CALUX Cell Line | Estrogenic activity screening | Reporter gene assay detecting ERα activation | Measuring potency of bisphenols, parabens, phthalates [97] |
| PPARγ Reporter Assays | Metabolic disruption screening | Identify activators of PPARγ pathway | Assessing adipogenic potential of chemicals like TPP [99] |
| LC-MS/MS Systems | Biomarker quantification | Precise measurement of EDCs and metabolites in biological samples | Quantifying phthalate metabolites in urine [12] |
| Silicon Microphysiological Systems (Organs-on-chip) | Advanced tissue modeling | Simulate human organ-level responses without animal models | Studying multi-tissue interactions of EDCs [96] [98] |
| NHANES Dataset | Epidemiological analysis | Population-level exposure and health data | Investigating EDC-asthma relationships [15] [12] |
| Adverse Outcome Pathway (AOP) Framework | Mechanistic toxicology | Organize knowledge from molecular initiation to adverse outcomes | Structured assessment of human relevance [98] |
Signaling Pathways and Mechanistic Insights in EDC Research
EDCs can interfere with multiple hormonal pathways, but their effects primarily manifest through specific molecular initiating events and downstream key events. The following diagram illustrates the major signaling pathways disrupted by EDCs and their connection to adverse outcomes:
Figure 2: EDC Mechanisms: Molecular Initiating Events to Adverse Outcomes
Advanced WoE Application: Human Relevance Assessment Framework
A critical application of WoE in EDC research is determining the human relevance of adverse outcome pathways (AOPs) identified primarily in experimental systems. A refined workflow for this assessment has been developed building on the WHO/IPCS Mode of Action framework [98]:
Human Relevance Assessment Workflow
The human relevance assessment follows a structured approach with key decision points:
Figure 3: Human Relevance Assessment Workflow for AOPs
Weight of Evidence Integration for Human Relevance
The final step in human relevance assessment involves integrating different lines of evidence using a WoE approach [98]. This includes:
Biological Evidence Considerations:
- Evolutionary conservation of molecular targets across species
- Tissue distribution and expression patterns of key receptors
- Functional competence of pathway components in human systems
Empirical Evidence Considerations:
- Epidemiological data linking exposure to adverse outcomes in humans
- In vitro studies using human cells and tissues
- Concordance between human and animal responses for similar endpoints
NAM Relevance Assessment:
- Evaluation of whether New Approach Methodologies adequately represent human biology
- Consideration of metabolic competence, cellular context, and physiological relevance
- Assessment of predictive capacity for human outcomes
This structured approach to human relevance assessment is particularly important for EDCs, where effects may be species-specific, life stage-dependent, and exhibit non-monotonic dose responses [98] [70].
The Weight of Evidence framework provides an essential methodology for integrating heterogeneous data from epidemiological and toxicological studies on EDC exposure through food, air, and skin routes. By systematically evaluating study quality, relevance to real-world exposure scenarios, and consistency across different methodological approaches, researchers can develop more robust conclusions about EDC risks while avoiding overreactions to isolated or sensational findings [96].
The continuing refinement of WoE methodologies—including formal expert knowledge elicitation [99], advanced mixture analysis techniques [15], and structured human relevance assessment [98]—promises to enhance the scientific basis for regulatory decisions and public health protection regarding endocrine-disrupting chemicals.
Comparative Analysis of Global Regulatory Standards (EPA, EU, WHO)
Environmental endocrine-disrupting chemicals (EDCs) represent a significant and growing challenge to global public health, with exposure routes primarily through food, air, and skin absorption posing particular threats to reproductive, metabolic, and developmental systems. These synthetic and naturally occurring substances interfere with normal hormonal signaling pathways, with mounting evidence suggesting they play a pivotal role in the global decline of male fertility and other health endpoints [14]. The regulation of these chemicals varies considerably across major international bodies, creating a complex landscape for researchers, risk assessors, and public health professionals.
This technical guide provides a comparative analysis of the regulatory frameworks established by the United States Environmental Protection Agency (EPA), the European Union (EU), and the World Health Organization (WHO). Framed within the context of ongoing research on EDC exposure routes, this review aims to elucidate the similarities, differences, and evolving methodologies that define global approaches to managing the risks posed by these ubiquitous environmental contaminants. Understanding these regulatory paradigms is essential for designing robust studies, interpreting toxicological data, and developing effective public health interventions.
The regulatory approaches to EDCs are shaped by regional legal traditions, scientific advisory mechanisms, and risk tolerance levels. The following analysis delineates the core structures and recent developments within the EPA, EU, and WHO frameworks.
United States Environmental Protection Agency (EPA) Framework
The US EPA's approach to regulating endocrine disruptors has undergone significant evolution and, more recently, a strategic reconsideration of its foundational policies.
Regulatory Foundations and Recent Shifts: The EPA's authority to regulate greenhouse gases, which intersects with the control of certain EDCs, was historically based on the 2009 Endangerment Finding [100]. This finding allowed the agency to classify six greenhouse gases as threats to public health and welfare. However, in a major policy shift, the EPA announced in March 2025 plans to launch a formal reconsideration of this landmark finding [100]. This move signals a potential wholesale revision of the legal basis for climate and related chemical regulations in the US. Concurrently, the agency is reconsidering the Greenhouse Gas Reporting Program, which requires over 8,000 facilities to report emissions annually [100].
Chemical Assessment and Testing: The EPA's Endocrine Disruptor Screening Program (EDSP) employs a two-tiered framework to identify potential endocrine disruptors. Tier 1 consists of assays to detect interaction with estrogen, androgen, and thyroid systems, while Tier 2 involves longer-term tests to assess adverse effects. The program has faced challenges related to the pace of testing and the integration of new scientific methodologies.
State-Level Initiatives: In the absence of consistent federal momentum, individual states have taken proactive measures. For instance, the New York State Department of Environmental Conservation (DEC) proposed regulations in March 2025 to require power plants, natural gas suppliers, and landfills emitting over 10,000 metric tons of CO2e to report their emissions [100]. Similarly, California has enacted laws (SB 253 and SB 261) mandating comprehensive climate-related disclosures from large companies, though these face ongoing legal challenges [101].
European Union (EU) Regulatory Framework
The European Union has established one of the world's most comprehensive and precautionary regulatory systems for chemicals, including EDCs, characterized by a dense network of intersecting directives and regulations.
Precautionary Principle and Holistic Legislation: The EU's approach is fundamentally guided by the precautionary principle, which permits restrictive measures even in the face of scientific uncertainty. This is embedded within broader policy frameworks like the European Green Deal, which aims for climate neutrality by 2050 [101]. Key regulatory pillars include REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals), which addresses the production and use of chemical substances, and the CLP Regulation (Classification, Labelling and Packaging), which ensures the hazards of chemicals are clearly communicated.
Sustainability Reporting and Due Diligence: A defining feature of the EU's strategy is its focus on corporate accountability and transparency. The Corporate Sustainability Reporting Directive (CSRD) significantly expands obligations for companies to disclose environmental and social impacts [100] [102]. While the European Council has moved to delay implementation for some companies by two years as part of its "Omnibus Simplification Package," the core framework remains [100]. Complementing this is the Corporate Sustainability Due Diligence Directive (CSDDD), which mandates that companies identify, prevent, and mitigate environmental and human rights impacts in their global value chains [101].
Sector-Specific Measures and the EU Taxonomy: The EU employs targeted regulations for specific sectors and exposure pathways. The EU Taxonomy provides a classification system for sustainable economic activities, guiding investment [102]. Furthermore, regulations governing plant protection products and biocides explicitly include criteria for the identification of endocrine-disrupting properties, leading to restrictions or bans on substances that meet these criteria.
World Health Organization (WHO) and Global Initiatives
The WHO's role differs from the legislative authority of the EPA or EU, focusing instead on global assessment, capacity building, and the establishment of scientific norms.
Assessment and Normative Guidance: The WHO, often in partnership with the United Nations Environment Programme (UNEP), has produced state-of-the-science reports on EDCs, such as the "State of the Science of Endocrine Disrupting Chemicals." These documents synthesize global evidence, identify research gaps, and raise awareness among member states. The WHO also establishes health-based guideline values for chemicals in drinking water and air, which serve as critical references for national governments setting their own standards.
Capacity Building and Harmonization: A key function of the WHO is to strengthen the capacity of low- and middle-income countries to assess and manage chemical risks. This includes developing toolkits for biomonitoring and risk assessment. The WHO also contributes to global harmonization efforts, such as those led by the International Programme on Chemical Safety (IPCS) and the Organisation for Economic Co-operation and Development (OECD), which work to align test guidelines and hazard assessment methodologies for EDCs across countries.
The International Sustainability Standards Board (ISSB): While not part of the WHO, the ISSB represents a critical global initiative for standardizing sustainability disclosures. In March 2025, the ISSB launched a jurisdictional roadmap tool to guide the adoption of its sustainability standards globally [100]. This tool helps jurisdictions set objectives and measure progress, which can indirectly influence how companies report on chemical management and EDC-related risks.
Table 1: Comparative Overview of Key Regulatory Frameworks for EDCs
| Aspect | U.S. EPA | European Union | WHO/Global Initiatives |
|---|---|---|---|
| Guiding Principle | Risk-based assessment, with recent shifts toward deregulation [100] [101] | Precautionary Principle [101] [102] | Public health protection, capacity building |
| Key Regulatory Instruments | Endangerment Finding (under reconsideration) [100], EDSP, TSCA | REACH, CLP, Plant Protection Products Regulation, Biocidal Products Regulation | WHO Guideline Values, IPCS/OECD Test Guidelines |
| Reporting & Transparency | GHG Reporting Program (under reconsideration) [100]; state-level laws (e.g., CA) [101] | CSRD, CSDDD, EU Taxonomy [100] [101] [102] | ISSB standards for jurisdictional adoption [100] |
| Focus on Exposure Routes | Considers pathways in chemical assessments | Explicit in regulations (e.g., product-specific directives) | Integrated into risk assessment guidance documents |
| Primary Authority | Regulatory and enforcement | Regulatory and enforcement | Scientific, normative, and advisory |
Experimental Protocols for EDC Research
Research into the health effects of EDCs relies on a multi-faceted methodology that spans from molecular analysis to population-level studies. The following protocols are essential for investigating the impact of EDCs absorbed via food, air, and skin.
Biomarker Assessment and Exposure Quantification
Accurately measuring internal exposure is a critical first step in linking EDCs to health outcomes.
Specimen Collection and Biomonitoring: The primary matrices for human biomonitoring include urine, blood, and serum. For instance, phthalate metabolites are frequently measured in urine, while persistent organic pollutants like PCBs and PBDEs are analyzed in serum due to their lipophilic nature [14]. Hair and saliva can serve as non-invasive alternatives for certain analytes [13]. Collection must follow strict protocols to avoid contamination (e.g., using phthalate-free gloves and collection vessels) and ensure sample stability (e.g., freezing at -20°C to -80°C).
Chemical Analysis Techniques: High-performance liquid chromatography (HPLC) coupled with tandem mass spectrometry (MS/MS) is the gold standard for quantifying a broad spectrum of EDCs, including BPA, phthalates, and pesticides, at low concentrations (parts-per-billion or trillion). For metals like cadmium and lead, inductively coupled plasma mass spectrometry (ICP-MS) provides high-sensitivity detection.
Questionnaire-Based Exposure Assessment: To complement biomonitoring and assess specific exposure routes, validated surveys are employed. A 2025 study developed a 19-item questionnaire to assess reproductive health behaviors aimed at reducing EDC exposure through food (e.g., consumption of canned foods), respiration, and skin absorption (e.g., use of personal care products) [13]. Such tools use Likert scales to quantify the frequency of exposure-related behaviors.
In Vitro and Mechanistic Assays
These assays are used to identify the molecular mechanisms by which EDCs disrupt endocrine function.
Receptor Binding and Transactivation Assays: These experiments determine if a chemical can bind to and activate hormone receptors such as estrogen (ER), androgen (AR), or thyroid (TR) receptors. Yeast or mammalian cell lines are engineered to express a human hormone receptor and a reporter gene (e.g., luciferase). Cells are exposed to the test chemical, and receptor activation is quantified by measuring reporter gene activity. A significant increase in activity compared to controls indicates endocrine-disrupting potential.
Assessment of Steroidogenesis: The H295R steroidogenesis assay (an OECD guideline) uses a human adrenocortical carcinoma cell line to assess the impact of chemicals on the production of key hormones like testosterone and estradiol. Cells are exposed to the EDC, and hormone levels in the medium are measured using enzyme-linked immunosorbent assays (ELISA) or MS/MS. This protocol directly evaluates disruption of a critical hormonal pathway.
In Vivo and Epidemiological Studies
These studies are crucial for establishing a causal link between EDC exposure and adverse health outcomes in whole organisms and populations.
Controlled Animal Studies: Rodent models are the most common for assessing EDC effects. A typical protocol involves exposing animals (e.g., rats) to a range of EDC doses via a relevant route (oral, dermal, inhalation) during critical developmental windows. Endpoints examined include:
- Organ Weights: Changes in the weight of reproductive organs (testes, prostate, ovaries, uterus).
- Histopathology: Microscopic examination of tissue morphology.
- Sperm Analysis: Count, motility, and morphology [14].
- Gene and Protein Expression: Analysis of transcriptional and translational changes in hormone-related genes.
Human Epidemiological Studies: Cohort, case-control, and cross-sectional studies correlate measured EDC exposure levels with health outcomes. For example, studies have linked urinary levels of certain phthalates to decreased gestational age and increased risk of preterm birth [2], and diminished immune response to vaccines in children exposed to PFAS [2]. These studies must carefully control for confounders like age, diet, and socioeconomic status.
Table 2: Key Research Reagents and Materials for EDC Studies
| Item | Function/Application | Example Use |
|---|---|---|
| Certified Reference Standards | Calibrating analytical instruments and quantifying EDCs in samples. | Quantifying BPA, phthalate metabolites, or PFAS in serum/urine via LC-MS/MS. |
| ELISA Kits | Measuring concentrations of hormones, cytokines, or other proteins in biological fluids. | Assessing changes in testosterone or estradiol levels in cell culture media or serum. |
| Cell Lines (e.g., H295R, MCF-7) | Modeling human biological processes for mechanistic studies. | H295R for steroidogenesis assay; MCF-7 (breast cancer) cells for estrogenicity assays. |
| Specific Antibodies | Detecting and visualizing target proteins in tissues (IHC) or Western blots. | Staining for estrogen receptor alpha (ERα) in uterine tissue sections. |
| DNA Methylation Kits | Isolating and analyzing epigenetic modifications. | Investigating transgenerational inheritance of EDC effects by assessing sperm DNA methylation patterns. |
Visualization of EDC Research and Regulation
The following diagrams illustrate the core pathways of EDC disruption and the integrated workflow for regulatory risk assessment.
EDC Mechanisms of Action
EDC Mechanisms and Health Effects
Integrated Risk Assessment Workflow
Risk Assessment to Regulation
Discussion and Future Directions
The comparative analysis reveals a stark transatlantic divergence in regulatory philosophy and application. The EU's precautionary, comprehensive framework contrasts with the current US trend of deregulation and reconsideration of established policies [100] [101]. This divide presents significant challenges for multinational corporations and international research collaborations, necessitating sophisticated compliance strategies that can navigate conflicting legal requirements.
Critical knowledge gaps persist, particularly in understanding the effects of complex mixtures of EDCs, which more accurately reflect real-world exposure than single-chemical studies [14]. Furthermore, the phenomenon of non-monotonic dose responses (NMDRs), where low doses have potent effects, challenges traditional toxicological paradigms and requires new risk assessment models [14]. Perhaps the most profound frontier is the study of transgenerational epigenetic effects, where animal models show that EDC exposure can impair the reproductive health of subsequent generations through mechanisms like DNA methylation, though human evidence remains limited [14].
Future research must prioritize the development of high-throughput screening methods and computational toxicology models to efficiently evaluate the vast number of chemicals in commerce. Enhanced biomonitoring and the validation of sensitive epigenetic biomarkers will be crucial for early detection of EDC-related health risks. Ultimately, closing these scientific gaps is essential for informing evidence-based regulatory frameworks that are robust enough to protect human and environmental health across all exposure routes.
Validation of Behavioral Interventions for Exposure Mitigation
The increasing understanding of exposure risks from endocrine-disrupting chemicals (EDCs) through routes including food, air, and skin absorption has created an urgent need for effective exposure mitigation strategies [103]. Behavioral Intervention Technologies (BITs) represent evidence-based approaches that apply behavioral and psychological strategies through technological systems to support health behavior change [104] [105]. Within environmental health, these interventions can be systematically developed and validated to help individuals and communities reduce their exposure to environmental contaminants. This technical guide provides a comprehensive framework for validating behavioral interventions specifically designed for EDC exposure mitigation, presenting methodological rigor suitable for research scientists and drug development professionals working at the intersection of environmental toxicology and behavioral science.
Theoretical Foundations and Validation Frameworks
The NIH Stage Model for Behavioral Intervention Development
The NIH Stage Model provides a structured framework for developing and validating behavioral interventions through an iterative, recursive process [106]. This model is particularly valuable for environmental exposure mitigation as it emphasizes intervention potency and implementability from initial development through real-world implementation.
- Stage 0: Basic science research relevant to intervention development, including mechanisms of EDC exposure and human behavior patterns related to exposure risks.
- Stage I: Intervention generation, refinement, modification, and adaptation, culminating in feasibility and pilot testing for exposure reduction behaviors.
- Stage II: Efficacy testing under controlled research conditions to establish causal relationships between intervention components and exposure reduction outcomes.
- Stage III: Real-world efficacy testing in community settings while maintaining scientific control to establish internal validity.
- Stage IV: Effectiveness research examining interventions in real-world settings while maximizing external validity and relevance to diverse populations.
- Stage V: Implementation and dissemination research to translate validated interventions into widespread practice [106].
This framework ensures that behavioral interventions for exposure mitigation are not only efficacious but also readily implementable within target populations, with consideration for the intervention's ease of implementation encouraged as early as possible in the development process.
Behavioral Intervention Technologies (BITs) for Exposure Mitigation
BITs encompass behavioral and psychological interventions that use information and communication technology features to address health outcomes [104] [105]. For EDC exposure mitigation, relevant BIT approaches include:
- Web-based interventions that provide psychoeducation about exposure sources, self-monitoring tools for tracking mitigation behaviors, and personalized feedback on risk reduction.
- Mobile technologies that deliver just-in-time interventions when exposure risks are detected or anticipated, leveraging sensor data and ecological momentary assessments.
- Virtual reality environments that create simulated exposure scenarios for skills training in exposure identification and mitigation.
- Serious gaming approaches that engage users in immersive experiences for learning complex exposure avoidance behaviors [104].
These technologies provide valuable platforms for delivering standardized, scalable interventions while enabling rigorous data collection on both intervention engagement and exposure outcomes.
Methodological Framework for Validation
Defining Exposure and Outcome Metrics
Validating behavioral interventions for EDC exposure mitigation requires precise operationalization of both exposure metrics and behavioral outcomes. The U.S. EPA provides comprehensive frameworks for quantifying exposure through various routes that should inform outcome measurement in validation studies [107] [108].
Table 1: Key Exposure and Behavioral Outcomes for Intervention Validation
| Domain | Metric Category | Specific Measures | Data Collection Methods |
|---|---|---|---|
| Dermal Exposure | Internal Dose | Amount of contaminant absorbed through skin (mg/cm²-event) [107] | Permeability coefficients, skin wipes, biomonitoring |
| Average Daily Dose | ADD = DAevent × SA × EF × ED / (BW × AT) [107] | Calculated from environmental and behavioral data | |
| Ingestion Exposure | Potential Dose | Amount of contaminant ingested (mg/kg-day) [108] | Food diaries, environmental sampling, intake surveys |
| Applied Dose | Amount at gastrointestinal absorption barrier [108] | Bioaccessibility assays, biomonitoring | |
| Behavioral Outcomes | Exposure Avoidance | Reduction in use of products containing EDCs | Self-report, product use inventories, receipt scanning |
| Mitigation Behaviors | Increased protective behaviors (e.g., food washing, ventilation) | Direct observation, self-report, sensor data |
The Five A's Framework for Intervention Design
The U.S. Preventive Services Task Force recommends the Five A's construct as a framework for organizing behavioral counseling interventions [109]. This framework provides a systematic approach to designing exposure mitigation interventions:
- Assess: Identify individual exposure risks, current mitigation behaviors, and readiness to change.
- Advise: Provide clear, specific, personalized guidance on exposure mitigation strategies.
- Agree: Collaboratively set mitigation goals based on personal preferences and confidence.
- Assist: Aid in acquiring skills, tools, and social support for behavior change.
- Arrange: Schedule follow-up contacts to provide ongoing support and adjust plans [109].
This framework is particularly valuable for standardizing intervention components across validation studies, enabling clearer identification of active ingredients responsible for behavior change.
Experimental Designs for Validation
Rigorous validation of behavioral interventions for exposure mitigation requires appropriate experimental designs that balance internal and external validity:
- Stage II Efficacy Trials: Randomized controlled trials with research-based providers, standardized protocols, and controlled conditions to establish causal efficacy for exposure reduction [106].
- Stage III Hybrid Efficacy-Effectiveness Trials: Experimental testing in community settings with real-world providers while maintaining control sufficient for internal validity [106].
- Sequential Multiple Assignment Randomized Trials (SMART): Adaptive designs that allow intervention components to be tailored based on individual response patterns.
- Micro-Randomized Trials: Intensive longitudinal designs that randomize intervention components repeatedly within individuals to optimize just-in-time adaptive interventions.
Each design serves distinct validation objectives, with selection dependent on the intervention's stage of development and specific research questions regarding efficacy, effectiveness, or implementation.
Experimental Protocols and Assessment Methods
Protocol for Validating Dermal Exposure Mitigation Interventions
Dermal exposure represents a significant pathway for EDC uptake, particularly for chemicals such as phthalates and bisphenols found in personal care products and household environments [107] [103]. The following protocol provides a standardized approach for validating behavioral interventions targeting dermal exposure reduction:
Objective: To evaluate the efficacy of a BIT-based intervention for reducing dermal absorption of EDCs from personal care products and household environments.
Participant Recruitment and Screening:
- Recruit adult participants (n=200 minimum) with verified use of ≥2 personal care products containing known EDCs (e.g., phthalates, parabens).
- Include both sexes, with oversampling of populations with potentially higher vulnerability (e.g., pregnant women, adolescents).
- Exclude individuals with occupational EDC exposure to isolate consumer product effects.
Baseline Assessment:
- Collect urine samples for biomonitoring of EDC metabolites (e.g., MEP, MiBP, BPA) using LC-MS/MS quantification.
- Administer comprehensive exposure behavior inventory documenting product use, household practices, and potential exposure sources.
- Measure skin surface area potentially exposed during typical product application using standardized body surface area calculations [107].
Intervention Components:
- Core Education Module: Web-based tutorial on EDC sources in personal care products and household environments, with specific training on product label reading.
- Self-Monitoring Tool: Mobile application for tracking product use and potential exposure events with real-time feedback.
- Alternative Product Guidance: Personalized recommendations for EDC-free alternatives based on individual product preferences and budget.
- Support Component: Weekly videoconferencing sessions with trained coaches using motivational interviewing techniques to address implementation barriers [104] [109].
Outcome Assessment:
- Primary Outcome: Change in urinary EDC metabolite concentrations from baseline to 3-month and 6-month follow-ups.
- Secondary Outcomes:
- Reduction in use of EDC-containing products verified by product inventories and receipt data.
- Increase in protective behaviors (e.g., glove use during cleaning, reduced use of fragranced products) measured by self-report and direct observation.
- Calculated reduction in potential dermal dose using EPA algorithms: DAevent = Kp × C × t [107].
Data Analysis Plan:
- Intent-to-treat analysis using linear mixed models to examine group × time interactions on urinary metabolite levels.
- Mediation analysis to test whether behavior changes explain intervention effects on biomarker outcomes.
- Dose-response analysis examining intervention engagement metrics in relation to exposure reduction.
Protocol for Dietary Exposure Mitigation Intervention
Dietary intake represents a major exposure pathway for numerous EDCs, including bisphenols from food packaging and organochlorine pesticides from conventional produce [103] [108]. This protocol validates interventions targeting dietary exposure reduction:
Objective: To test the efficacy of a technology-supported behavioral intervention for reducing dietary exposure to EDCs through food selection and preparation practices.
Participant Characteristics:
- Recruit primary household food shoppers (n=150 minimum) with responsibility for ≥75% of household food purchases.
- Target households with children <18 years to examine family-level exposure reduction.
- Include diverse socioeconomic strata to examine intervention effects across resource levels.
Baseline Dietary Exposure Assessment:
- Collect spot urine samples for biomonitoring of dietary EDC biomarkers (BPA, phthalates, OP pesticides).
- Conduct 3-day dietary recalls with photographic documentation of meals and packaging.
- Inventory household food storage and preparation materials for EDC leaching potential.
Intervention Components:
- Food Selection Training: Virtual reality simulation of grocery shopping with real-time feedback on high-risk and lower-risk food choices.
- Personalized Alert System: Mobile notifications when shopping in stores, providing alternative suggestions for high-EDC food items identified in baseline assessment.
- Food Preparation Training: Video demonstrations of exposure-reducing preparation techniques (e.g., peeling, thorough washing, fat trimming).
- Social Support Component: Private social media group for participants to share strategies, recipes, and successes [104].
Outcome Measures:
- Primary Outcome: Change in urinary BPA and DEHP metabolites from baseline to 3-month and 6-month follow-ups.
- Secondary Outcomes:
- Increased purchase of organic produce (verified by receipt analysis).
- Reduction in canned food consumption (measured by pantry inventories and shopping data).
- Improved food preparation practices (assessed through video submissions of meal preparation).
- Calculated reduction in average daily dose using EPA algorithm: ADD = Cmedium × IngR × EF × ED / (BW × AT) [108].
Statistical Analysis:
- Multilevel modeling to account for household clustering of participants.
- Path analysis to test theoretical mechanisms of behavior change.
- Cost-effectiveness analysis comparing intervention costs to exposure reduction benefits.
Data Analysis and Interpretation
Quantitative Analysis of Intervention Effects
Robust statistical analysis is essential for establishing intervention efficacy and understanding mechanisms of action:
- Primary Efficacy Analysis: Intent-to-treat analysis comparing intervention and control groups on biomarker and behavioral outcomes using appropriate generalized linear models.
- Mediation Analysis: Testing whether hypothesized behavior changes mediate intervention effects on exposure biomarkers using path analysis or structural equation modeling.
- Moderation Analysis: Examination of participant characteristics (e.g., socioeconomic status, baseline exposure levels, technological proficiency) as moderators of intervention effects.
- Dose-Response Relationships: Analysis relating intervention engagement metrics (e.g., logins, module completions, coach sessions attended) to exposure reduction outcomes.
Interpretation of Biomarker Data
Biomonitoring data provide critical objective evidence of exposure reduction but require careful interpretation:
- Account for temporal variability in EDC biomarkers through repeated measures and careful timing of specimen collection relative to exposure events.
- Consider pharmacokinetics when interpreting biomarker concentrations in relation to intervention timing, particularly for short-half-life compounds.
- Use creatinine-corrected values or specific gravity adjustments to account for urine dilution.
- Interpret effect sizes in context of population health relevant reductions, considering non-linear dose-response relationships for many EDCs.
Table 2: Key EDC Biomarkers for Intervention Validation
| EDC Class | Specific Compounds | Primary Exposure Routes | Validation Challenges |
|---|---|---|---|
| Bisphenols | BPA, BPS, BPF | Food packaging, canned foods, receipts [103] | Short half-life requires precise timing of specimen collection |
| Phthalates | DEHP, DiNP, MiBP | Personal care products, food packaging, vinyl products [15] [103] | Ubiquitous exposure creates high background; multiple metabolites |
| Organochlorine Pesticides | DDT, HCH | Diet (lipid-rich foods), historical environmental contamination [103] | Long half-lives limit sensitivity to detect recent behavior change |
| PFAS | PFOA, PFOS | Food, drinking water, dust [103] | Complex exposure pathways; extremely long half-lives |
Visualization of Research Framework
Behavioral Intervention Validation Workflow
Multi-Route Exposure Assessment Framework
Table 3: Essential Reagents and Materials for Exposure Mitigation Research
| Category | Item | Specifications | Research Application |
|---|---|---|---|
| Biomonitoring | LC-MS/MS Systems | High-resolution capability for EDC metabolites | Quantification of exposure biomarkers in biological specimens |
| Stable Isotope-Labeled Internal Standards | 13C- or 2H-labeled EDC analogs | Correction for analytical recovery in biomarker quantification | |
| Solid Phase Extraction Cartridges | C18 or mixed-mode sorbents | Sample cleanup and preconcentration for biomarker analysis | |
| Environmental Sampling | Passive Samplers | Silicone wristbands, PUF-PAS | Personal exposure assessment for airborne EDCs |
| Wipe Sampling Kits | Ghostwipes, cellulose filters | Surface contamination measurement for dermal exposure estimation | |
| Composite Food Sampling | Glass containers, Teflon lids | Dietary exposure assessment from food commodities | |
| Behavioral Assessment | Ecological Momentary Assessment | Mobile app platforms with push notifications | Real-time behavior tracking in natural environments |
| Product Ingredient Databases | FDA, EPA, proprietary databases | Identification of EDCs in consumer products | |
| Geospatial Tracking | GPS loggers, smartphone location services | Mapping activity patterns related to exposure hotspots | |
| Intervention Delivery | BIT Platforms | Custom web applications, mobile apps | Standardized intervention delivery with usage analytics |
| Videoconferencing Systems | HIPAA-compliant platforms (e.g., Zoom for Healthcare) | Remote coaching sessions with secure communication | |
| Sensor Technologies | Wearable activity trackers, environmental sensors | Objective monitoring of mitigation behaviors and contexts |
The validation of behavioral interventions for EDC exposure mitigation requires methodologically rigorous approaches that integrate environmental exposure science with behavioral intervention research. The framework presented in this technical guide enables researchers to systematically develop, test, and implement interventions that effectively reduce exposure through dermal, ingestion, and inhalation routes. Future research should prioritize several key areas:
- Personalized Intervention Approaches: Development of adaptive interventions that tailor mitigation strategies based on individual exposure profiles, genetic susceptibility, and behavioral preferences.
- Advanced Measurement Technologies: Integration of novel sensor technologies and omics approaches for more comprehensive exposure assessment and intervention response monitoring.
- Implementation Science: Systematic study of strategies for implementing validated exposure mitigation interventions in diverse real-world settings, including clinical care, public health programs, and community organizations.
- Chemical Mixture Considerations: Addressing the challenges of validating interventions for complex mixtures of EDCs that occur in real-world exposure scenarios.
- Long-Term Efficacy Studies: Examination of intervention effects over extended timeframes to establish durability of exposure reduction and potential health impacts.
As evidence accumulates regarding the health consequences of EDC exposure, validated behavioral interventions will play an increasingly important role in exposure mitigation strategies alongside regulatory and environmental controls.
Benchmarking EDC Identification Processes Across International Agencies
Endocrine Disrupting Chemicals (EDCs) represent a significant public health concern due to their ability to interfere with hormonal systems. The identification and assessment of EDCs by regulatory agencies globally rely on understanding their primary exposure routes: food, air, and dermal absorption. These exposure pathways determine how EDCs enter biological systems, influencing both toxicological assessments and regulatory decision-making. The complexity of EDC effects, which may manifest at extremely low doses with non-monotonic dose responses, necessitates sophisticated identification frameworks that account for exposure route-specific pharmacokinetics and pharmacodynamics.
International regulatory agencies have developed distinct but overlapping approaches to EDC identification, reflecting different legal frameworks, scientific traditions, and risk assessment philosophies. This technical guide provides researchers and drug development professionals with a comprehensive benchmarking analysis of these processes, with particular emphasis on the experimental methodologies that support route-of-exposure investigations. Understanding these frameworks is essential for designing compliant toxicological studies and advancing research on EDC impacts on human health.
Core Principles of EDC Exposure and Toxicokinetics
Primary Exposure Routes and Absorption Dynamics
EDCs enter the human body through three principal exposure routes, each with distinct absorption mechanisms that influence their bioavailability and toxicological potential:
Dermal Absorption: Chemicals contact skin and must penetrate the stratum corneum barrier to reach systemic circulation. The process is governed by Fick's Law of Diffusion, where flux across this homogeneous membrane is proportional to the concentration difference between outer and inner surfaces and inversely proportional to membrane thickness [107]. Key parameters affecting dermal absorption include the permeability coefficient (Kp), contaminant concentration in the contacting vehicle, and exposure duration [107]. The stratum corneum forms the primary barrier, consisting of 10-15 rows of highly keratinized corneocytes embedded in a lipid matrix, structurally resembling a "bricks and mortar" model [110].
Inhalation Exposure: Volatile organic compounds (VOCs) and semi-VOCs enter the respiratory system through inhaled air. These compounds possess boiling points between 50-100°C and 240-260°C respectively, affecting their distribution between air and body surfaces [111]. BTEX compounds (benzene, toluene, ethylbenzene, and xylenes) comprise over 60% of VOCs found in urban areas and serve as key reference compounds for environmental EDC exposure assessment [111].
Ingestion Exposure: EDCs in food and water pass through the gastrointestinal tract, where their absorption is influenced by chemical stability in digestive fluids, transport across intestinal mucosa, and first-pass metabolism in the liver. Inflammatory states can significantly alter absorption kinetics for ingested EDCs, as demonstrated by correlations between C-reactive protein levels and systemic exposure to various chemicals [112].
Toxicokinetic Modeling Approaches
Computational models are essential tools for predicting EDC distribution following different exposure routes. Compartmental pharmacokinetic models treat the skin and other absorption barriers as one or more well-stirred compartments with transfer between compartments depicted by first-order rate constant expressions [113]. These models must account for the multi-layer structure of absorption barriers - for skin, this includes the stratum corneum, viable epidermis, and dermis, each with distinct physicochemical properties affecting chemical transfer [113].
More sophisticated physiologically-based pharmacokinetic (PBPK) models incorporate actual physiological and biochemical parameters, including blood flow rates to tissues, tissue volumes, and metabolic pathways. These models are particularly valuable for extrapolating across exposure routes and species, and for predicting internal dose metrics relevant to endocrine disruption mechanisms.
Table 1: Key Parameters in Dermal Absorption Models
| Parameter | Description | Impact on Absorption |
|---|---|---|
| Permeability Coefficient (Kp) | Chemical-specific diffusion rate across skin | Directly determines absorption rate |
| Stratum Corneum Thickness | Varies by anatomical site, age, and species | Thinner barriers increase absorption |
| Partition Coefficient | Lipid-to-water solubility ratio | Optimal range (typically 1-3) enhances penetration |
| Molecular Weight | Size of chemical molecule | Molecules <500 Da penetrate more readily |
| Vehicle Effects | Formulation containing the chemical | Can dramatically alter stratum corneum permeability |
| Exposure Duration | Contact time with skin | Longer exposure increases total absorption |
International Regulatory Frameworks for EDC Assessment
Biomarker Qualification and Context of Use
Regulatory agencies employ structured frameworks for evaluating scientific evidence supporting EDC identification. The U.S. Food and Drug Administration's Biomarker Qualification Program (BQP) provides a pathway for regulatory acceptance of biomarkers for specific contexts of use (COU) in drug development [114]. This program involves three stages: Letter of Intent, Qualification Plan, and Full Qualification Package, establishing a standardized approach for assessing biomarkers relevant to EDC effects.
The BEST (Biomarkers, EndpointS, and other Tools) Resource framework developed through FDA-NIH collaboration categorizes biomarkers into several types [114]:
- Susceptibility/Risk Biomarkers: Identify individuals with increased probability of developing endocrine-related disorders
- Diagnostic Biomarkers: Detect and confirm endocrine disruption conditions
- Monitoring Biomarkers: Assess EDC exposure status or response to interventions over time
- Predictive Biomarkers: Identify likelihood of differential response to EDC exposure
- Pharmacodynamic/Response Biomarkers: Indicate biological responses to EDC exposure
- Safety Biomarkers: Detect toxic effects before irreversible damage occurs
Comparative Agency Approaches
International regulatory agencies share common principles in EDC assessment while maintaining distinct procedural frameworks:
U.S. Environmental Protection Agency (EPA): Utilizes a tiered testing approach that progresses from rapid in vitro assays to comprehensive in vivo studies. The EPA's Exposure Assessment Tools include specific methodologies for dermal route evaluation, accounting for skin surface area, adherence factors, and transfer efficiencies [107]. For volatile EDCs, the EPA's National Emissions Inventory (NEI) tracks emissions of hazardous air pollutants, establishing reference concentrations for risk assessments [111].
European Chemicals Agency (ECHA): Operates under the REACH regulation, requiring extensive data on substance properties, uses, and exposures. ECHA emphasizes the identification of Substances of Very High Concern (SVHC), including endocrine disruptors, through comprehensive dossiers and community rolling action plans (CoRAP).
OECD Test Guidelines: Provide internationally standardized testing methodologies relevant to EDC identification, including OECD 427 (Skin Absorption: In Vivo Method), OECD 428 (Skin Absorption: In Vitro Method), and OPPTS 870.7600 (Dermal Penetration) [115]. These guidelines ensure consistency in data generation across international jurisdictions.
The growing emphasis on Real-World Evidence (RWE) is influencing regulatory approaches to EDC assessment, with agencies increasingly considering data from electronic health records, patient-reported outcomes, claims databases, and environmental monitoring to complement traditional toxicological studies [116].
Table 2: International Regulatory Approaches to EDC Assessment
| Agency/Program | Key Guidance/Document | Testing Emphasis | Exposure Route Considerations |
|---|---|---|---|
| U.S. EPA | EDSP Tiered Testing | In vivo endocrine-specific | Dermal exposure algorithms for risk assessment |
| EU ECHA | REACH Annexes | In vitro screening + mechanistic | Exposure scenario development for all routes |
| OECD | Test Guidelines 427, 428 | Standardized protocols | Defined dermal absorption methodologies |
| FDA | Biomarker Qualification | Clinical relevance | Biomarker context of use for exposure effects |
Experimental Methodologies for EDC Identification
In Vitro Dermal Absorption Protocols
In vitro dermal absorption studies provide critical data on EDC penetration through human skin without requiring animal testing. The OECD Guideline 428 outlines standardized methodologies using static (Franz) cells or flow-through diffusion cells [115]. The experimental workflow involves:
Skin Preparation: Human or animal skin specimens are dermatomed to consistent thickness (typically 200-500 μm) and inspected for integrity using transepidermal water loss (TEWL) measurements or tritiated water permeability assays.
Test System Assembly: Skin sections are mounted between donor and receptor chambers with the stratum corneum facing the donor compartment. The receptor chamber contains physiologically relevant buffer (typically PBS with antimicrobial agents) maintained at 32°C to simulate skin temperature.
Test Substance Application: Radiolabeled or non-radiolabeled EDC preparations are applied to the donor chamber at environmentally relevant doses (typically 1-5 mg/cm²) in appropriate vehicles. Mass balance studies require radiolabeled test materials for precise quantification.
Sampling and Analysis: Receptor fluid is sampled at predetermined intervals over 24-48 hours. At termination, the skin is cleansed to remove unabsorbed test substance, then solubilized or tape-stripped to quantify EDC distribution across skin layers.
The absorbed dose is calculated as: DAevent = Kp × C × t where DAevent is the absorbed dose (mg/cm²-event), Kp is the permeability coefficient (cm/hr), C is the concentration of chemical contacting skin (mg/cm³), and t is contact time (hours/event) [107].
Biomarker Validation Methodologies
Biomarker development for EDC assessment follows a "fit-for-purpose" validation approach, where the level of evidence required depends on the specific context of use [114]. The validation framework encompasses:
Analytical Validation: Assessing biomarker measurement performance characteristics including accuracy, precision, analytical sensitivity, analytical specificity, reportable range, and reference range. For EDCs targeting specific endocrine pathways, this may involve developing ultra-sensitive LC-MS/MS assays capable of detecting hormonally active compounds at picomolar concentrations.
Clinical Validation: Demonstrating that the biomarker accurately identifies or predicts clinical outcomes of interest. This involves determining sensitivity, specificity, and positive/negative predictive values in the intended population. For EDCs, this may include establishing correlation between biomarker levels and functional endocrine outcomes across relevant exposure routes.
Context of Use Definition: Precisely specifying the biomarker's application in EDC assessment, such as diagnostic identification, exposure monitoring, or predictive toxicology. This definition drives the validation requirements and acceptance criteria.
In Vivo Absorption, Distribution, Metabolism, and Excretion (ADME) Studies
Comprehensive ADME studies characterize the systemic disposition of EDCs following different exposure routes. These studies typically employ radiolabeled compounds to enable precise mass balance calculations and metabolite identification. Key methodological considerations include:
Dosing Regimen Design: Route-specific administration techniques that mimic human exposures, including dietary admixture (ingestion), controlled atmosphere chambers (inhalation), and occluded or non-occluded dermal application.
Tissue Distribution Assessment: Quantitative whole-body autoradiography or tissue combustion techniques to determine EDC accumulation in endocrine-relevant tissues such as thyroid, adrenals, reproductive organs, and adipose tissue.
Metabolite Profiling: Identification and quantification of biotransformation products using high-resolution mass spectrometry to understand metabolic activation or detoxification pathways.
Excretion Balance: Comprehensive collection of urine, feces, and potentially expired air to establish mass balance and major elimination pathways.
The emerging focus on inflammatory biomarkers in pharmacokinetic studies is particularly relevant to EDC assessment, as systemic inflammation significantly alters chemical absorption, distribution, metabolism, and excretion - especially in vulnerable populations [112].
Advanced Research Technologies and Computational Approaches
Innovative Biomarker Technologies
Artificial intelligence and advanced analytics are transforming EDC identification through novel biomarker development:
AI-Powered Biomarkers: Machine learning algorithms analyze complex datasets to identify novel biomarker signatures of EDC exposure and effect. These approaches can integrate multi-omics data (transcriptomics, proteomics, metabolomics) with traditional toxicological endpoints to discover route-specific exposure signatures [116].
Digital Biomarkers: Wearable sensors and devices provide continuous, objective measurements of physiological and behavioral parameters relevant to endocrine function. These technologies enable real-world exposure assessment and effect monitoring outside controlled laboratory settings [116].
Synthetic Control Arms: Advanced trial methodologies using AI-generated control groups reduce the need for placebo cohorts in EDC intervention studies, addressing ethical concerns while maintaining statistical power [116].
Next-Generation Experimental Models
Advanced in vitro and in silico systems are enhancing the physiological relevance of EDC screening:
Microphysiological Systems (Organs-on-Chips): Microfluidic devices containing living human cells that simulate tissue-level and organ-level physiology. These systems permit route-specific exposure modeling and inter-tissue communication assessment relevant to endocrine disruption.
3D Tissue Models: Reconstructed human epidermis and full-thickness skin models that better replicate in vivo barrier properties and metabolic capacity compared to traditional monolayer cultures.
In Silico Prediction Tools: Quantitative Structure-Activity Relationship (QSAR) models that predict EDC potential based on chemical structural features, and PBPK models that simulate route-specific disposition kinetics.
Visualization of EDC Identification Workflows
Integrated EDC Assessment Framework
The following diagram illustrates the comprehensive workflow for identifying and assessing EDCs across exposure routes within regulatory frameworks:
Diagram 1: Integrated EDC Assessment Framework
Dermal Absorption Experimental Protocol
The following diagram details the standard experimental workflow for assessing dermal absorption of EDCs:
Diagram 2: Dermal Absorption Experimental Workflow
Research Reagent Solutions for EDC Studies
Table 3: Essential Research Reagents for EDC Identification Studies
| Reagent Category | Specific Examples | Research Application | Technical Considerations |
|---|---|---|---|
| In Vitro Test Systems | Reconstructed human epidermis (EpiDerm, EpiSkin), Organ-on-chip platforms | Dermal penetration studies, route-specific toxicity screening | Barrier function validation essential; match model to research question |
| Biomarker Assays | C-reactive protein kits, cytokine panels, hormone immunoassays, oxidative stress markers | Assessing inflammatory status effects on EDC pharmacokinetics | Consider cross-reactivity; validate for specific sample matrices |
| Analytical Standards | Certified reference materials, stable isotope-labeled EDCs, metabolite standards | Quantitative analysis by LC-MS/MS, method development and validation | Purity certification essential; consider stability during storage |
| Cell-Based Assay Kits | Luciferase reporter gene assays, receptor binding kits, cytochrome P450 inhibition assays | Screening for endocrine activity, metabolic pathway identification | Include appropriate controls; verify assay specificity and sensitivity |
| Diffusion Cell Systems | Franz static diffusion cells, flow-through diffusion systems | In vitro dermal absorption measurements | Maintain physiological temperature; ensure proper mixing in receptor chamber |
| Molecular Biology Reagents | qPCR kits for endocrine-responsive genes, RNA/DNA extraction kits, transfection reagents | Mechanistic studies of EDC effects on gene expression | Optimize extraction efficiency; include reference genes for normalization |
The benchmarking analysis presented in this technical guide reveals both convergence and divergence in international approaches to EDC identification. While regulatory agencies share fundamental principles of toxicological assessment, differences in testing requirements, classification frameworks, and risk assessment methodologies present challenges for global chemical management. The increasing emphasis on pathway-based assessments and adverse outcome pathways represents a significant evolution in EDC identification, moving away from traditional apical endpoint evaluation toward mechanistic understanding.
Future developments in EDC assessment will likely include greater integration of novel approach methodologies (NAMs) such as high-throughput screening, computational toxicology, and microphysiological systems to reduce animal testing while enhancing human relevance. The growing role of real-world evidence and biomonitoring data will strengthen the connection between controlled laboratory studies and population-level EDC exposures across different routes. Additionally, international harmonization initiatives will continue to address jurisdictional differences in testing requirements and risk assessment paradigms.
For researchers and drug development professionals, understanding these evolving frameworks is essential for designing robust EDC studies that meet regulatory standards while advancing the science of endocrine disruption. The experimental methodologies and technical resources outlined in this guide provide a foundation for generating high-quality data that supports evidence-based decision-making in EDC identification and risk assessment across international regulatory agencies.
Endocrine-disrupting chemicals (EDCs) represent a paradigm-shifting challenge in toxicology and public health. These exogenous substances interfere with hormone action, thereby increasing the risk of adverse health outcomes including cancer, reproductive impairment, cognitive deficits, and metabolic diseases [4]. The global scientific community has documented an alarming increase in EDC-related health burdens, with recent umbrella reviews identifying 69 statistically significant harmful associations between EDC exposure and various health outcomes [8]. The defining feature of the contemporary EDC landscape is the continuous emergence of novel chemicals that defy traditional risk assessment frameworks. With approximately 2,000 new chemicals entering the market annually and over 70% of the known 100,000 human-made chemicals lacking adequate endocrine activity testing [11], researchers face an unprecedented challenge in future-proofing their investigative approaches. This whitepaper provides a technical framework for advancing EDC research within the context of the precautionary principle, with particular emphasis on exposure routes—dietary, respiratory, and dermal—that mediate human contamination.
Emerging Chemicals of Concern: Beyond Traditional EDCs
Next-Generation Contaminants
While traditional EDCs like bisphenol A (BPA) and phthalates remain significant concerns, research must expand to include emerging compounds with insufficient safety data. The following table summarizes priority emerging EDCs based on recent evidence (2019-2025):
Table 1: Emerging Endocrine-Disrupting Chemicals of Concern
| Chemical Class | Primary Sources | Key Health Endpoints | Detection Challenges |
|---|---|---|---|
| Per- and polyfluoroalkyl substances (PFAS) [70] [11] | Fluorinated pesticides, waterproof coatings, fire-fighting foams | Earlier puberty, reduced fertility, PCOS, earlier menopause (by 1.9-3.8 years) [70] | Persistent in environment; bioaccumulative; multiple exposure routes |
| Novel plasticizers (e.g., BHPF) [71] | BPA-free plastics, food containers | Estrogen-antagonistic effects, reproductive toxicity | Marketed as "safe alternatives" but with similar endocrine activity |
| Metal oxide nanoparticles [27] | Industrial applications, consumer products | Sperm membrane damage, impaired motility (ED50: 150 mg/kg for TiO2) [27] | Nanoscale properties alter bioavailability and toxicity profiles |
| Brominated flame retardants (e.g., PBDEs) [27] | Electronics, furniture, textiles | Altered neurodevelopment, thyroid disruption, reproductive impairment | Environmental persistence (half-life: 3-7 years); high bioaccumulation potential (log Kow = 6.5-8.4) [27] |
| Phthalate alternatives (e.g., acetyl tributyl citrate) [11] | "Green" plastics, medical devices, food packaging | Metabolic disruption, developmental toxicity | Lack of robust toxicological databases; potential for pseudo-persistence |
Quantitative Associations Between EDC Classes and Health Outcomes
Recent comprehensive analyses have quantified the relationship between specific EDC classes and health outcomes, providing prioritization guidance for future research:
Table 2: EDC Exposure-Outcome Associations Based on Umbrella Review of 67 Meta-Analyses [8]
| EDC Category | Number of Cancer Outcomes | Neonatal/Child Outcomes | Metabolic Disorders | Cardiovascular Outcomes | Other Outcomes |
|---|---|---|---|---|---|
| Pesticides | 8 | 7 | 5 | 4 | 6 |
| BPA | 3 | 4 | 2 | 1 | 3 |
| PAHs | 5 | 3 | 4 | 3 | 3 |
| PFAS | 2 | 2 | 3 | 2 | 1 |
| Heavy Metals | 4 | 5 | 4 | 7 | 7 |
Regulatory Frameworks and the Precautionary Principle
Current Regulatory Landscape
Global regulatory approaches to EDC identification and management vary significantly, creating challenges for coherent research prioritization and chemical policy:
Table 3: Comparative Analysis of EDC Regulatory Identification Frameworks [11]
| Regulatory Body | Identification Approach | Key Criteria | Limitations |
|---|---|---|---|
| U.S. EPA EDSP | Weight-of-scientific-evidence | Unreasonable risk to health/environment under conditions of use | Slow testing process; limited to mandated chemicals |
| European Commission | Biological plausibility of causality | Adverse effects + endocrine mode of action in intact organisms | "Safe threshold" approach may not account for non-monotonic dose responses |
| WHO/UNEP | WHO definition (2012) + evidence synthesis | Interference with hormonal signaling + adverse health effects | Relies on existing assessments; limited proactive identification |
Application of the Precautionary Principle
The precautionary principle provides a critical framework for addressing scientific uncertainties surrounding emerging EDCs. Key applications in research include:
- Proactive Hazard Identification: Implementing the ten key characteristics of EDCs [4] as a screening tool for emerging chemicals before comprehensive toxicity data are available.
- Mixture Risk Assessment: Developing testing protocols that account for real-world "cocktail effects" of simultaneous exposure to multiple EDCs [11] [27], moving beyond single-chemical evaluation.
- Early Life Stage Focus: Prioritizing research on developmental exposures due to the enhanced susceptibility during critical windows of development [71] [4] and potential for transgenerational effects [27].
Mechanistic Insights: Key Characteristics and Signaling Pathways
Molecular Mechanisms of Endocrine Disruption
The consensus-based key characteristics framework provides a systematic approach for identifying EDC hazards through defined mechanistic pathways [4]:
Diagram 1: Key Characteristics of EDCs
EDC Interference with Reproductive Axis Signaling
Emerging research has elucidated specific pathway disruptions underlying EDC effects on reproductive health across the lifespan:
Diagram 2: EDC Effects on Reproductive Health
Experimental Approaches for Comprehensive EDC Assessment
Advanced Exposure Assessment Methodologies
Future-proofed EDC research requires innovative exposure assessment strategies that account for complex real-world exposure scenarios:
Integrated Exposure Assessment Protocol
- Multi-Matrix Biomonitoring: Simultaneous measurement of EDCs and their metabolites in paired samples (urine, blood, follicular fluid, breast milk, seminal plasma) to capture aggregate exposure [1] [70].
- Temporal Sampling Design: Collection of serial biospecimens across critical developmental windows (prenatal, early childhood, puberty, reproductive age) to assess exposure timing effects [71].
- Silent Spring Institute's RISE Questionnaire: Validated instrument for capturing exposure-related behaviors across dietary, respiratory, and dermal routes [60].
Mixture Exposure Simulation
- Proportionate Mixture Formulation: Prepare chemical mixtures reflecting real-world exposure proportions based on biomonitoring data rather than equipotent assumptions.
- Cumulative Risk Index Development: Weighted scoring system incorporating potency, exposure prevalence, and mixture interactions.
Mechanistic Bioassays for EDC Screening
The following tiered testing strategy provides comprehensive EDC hazard identification:
Table 4: Tiered Testing Strategy for EDC Hazard Identification
| Tier | Assay Type | Specific Methodologies | Endpoint Measurements |
|---|---|---|---|
| Tier 1: Receptor Interaction Screening | In vitro receptor activation | ERα, ERβ, AR, TR, GR, PR reporter assays | Receptor agonism/antagonism, dose-response curves, relative potency |
| Tier 2: Cellular Signaling Assessment | Cell-based functional assays | Steroidogenesis H295R assay, calcium signaling in α-cells [4], insulin signaling disruption assays | Hormone production, second messenger systems, gene expression changes |
| Tier 3: Complex System Evaluation | ex vivo models | Precision-cut tissue slices, primary hormone-producing cells, 3D organoid cultures | Tissue-specific responses, paracrine signaling, metabolic cooperation |
| Tier 4: Developmental & Transgenerational | In vivo life cycle exposure | Developmental exposure models, multi-generational cohort studies | Pubertal timing, gamete quality, epigenetic modifications, behavioral effects |
The Scientist's Toolkit: Essential Research Reagents and Platforms
Table 5: Essential Research Tools for EDC Investigation
| Tool Category | Specific Examples | Research Application | Technical Considerations |
|---|---|---|---|
| Biospecimen Collection | Silicone wristbands, hand wipes, dried blood spots | Non-invasive exposure assessment | Stability of analytes, extraction efficiency, background contamination |
| Analytical Chemistry | LC-MS/MS systems, HPLC-ESI-MS/MS, GC×GC-TOFMS | Quantification of EDCs and metabolites in complex matrices | Sensitivity (sub-ppb), isomer separation, matrix effects mitigation |
| Molecular Receptors | Stable reporter cell lines (ERα, AR, TRβ), membrane preparations | Receptor binding and activation screening | Cell line specificity, response elements, normalization controls |
| Epigenetic Tools | Methylated DNA immunoprecipitation, chromatin conformation capture | Transgenerational mechanism investigation | Tissue-specific patterns, computational analysis pipelines |
| Computational Resources | OECD QSAR Toolbox, EPA CompTox Chemistry Dashboard, AOP-Wiki | Priority setting and mixture risk assessment | Integration of experimental data, uncertainty quantification |
Research Gaps and Future Directions
Despite significant advances in EDC research, critical knowledge gaps persist that require prioritized investigation:
- Mixture Toxicology: Most studies focus on single-chemical exposures despite the reality of multi-chemical environmental exposure [11] [27]. Research must develop experimental designs that reflect real-world mixture exposures.
- Low-Dose and Non-Monotonic Effects: EDCs frequently exhibit non-monotonic dose-response (NMDR) patterns, where low-dose chronic exposure may produce more pronounced biological effects than acute high-dose exposure [27].
- Transgenerational Impacts: Animal studies demonstrate that EDC exposure can induce heritable epigenetic modifications affecting offspring fertility across multiple generations, but human epidemiological evidence remains limited [27].
- Sensitive Subpopulations: Research must better characterize vulnerability factors including genetic polymorphisms, pre-existing health conditions, and socioeconomic determinants of exposure.
- Advanced Biomarkers: Development of functional biomarkers beyond concentration measurements, including DNA methylation signatures, proteomic profiles, and metabolomic perturbations.
Future-proofing EDC research requires fundamental shifts in scientific approach, regulatory mindset, and public health protection. Researchers must adopt the precautionary principle as a operational framework rather than a philosophical concept, prioritizing preventive action in the face of uncertainty. This entails developing mechanism-based screening protocols that can identify potential EDCs before population-wide exposure occurs, implementing mixture assessment methodologies that reflect real-world exposure scenarios, and establishing transparent data sharing platforms that accelerate the identification of emerging concerns. By anchoring EDC research in the key characteristics framework [4] and adopting proactive assessment strategies, the scientific community can better protect public health against both known and emerging endocrine disruptors across the lifespan. The continued decline of global reproductive health indices [1] [18] [70] underscores the urgent need for this paradigm shift in chemical safety evaluation and environmental health protection.
Conclusion
Synthesizing the evidence across all intents reveals that EDC exposure through food, air, and skin presents a multifaceted challenge to human health, with particular implications for reproductive and cardiometabolic systems. A comprehensive approach—combining advanced biomonitoring, rigorous risk assessment that accounts for mixture effects and non-linear dose responses, and global regulatory alignment—is essential. Future research must prioritize elucidating transgenerational epigenetic effects, developing mechanism-based interventions, and integrating real-time exposure data into clinical and public health practice. For the biomedical community, this necessitates a shift towards more predictive toxicology and the development of strategies to mitigate EDC exposure as a critical component of therapeutic development and disease prevention.
References
- 1. Role of personal care products as endocrine disruptors ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12289576/]
- 2. Endocrine Disruptors [https://www.niehs.nih.gov/health/topics/agents/endocrine]
- 3. Global research on endocrine disruptors as emerging hazards ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12240779/]
- 4. Consensus on the key characteristics of endocrine ... [https://www.nature.com/articles/s41574-019-0273-8]
- 5. Early-life exposure to endocrine-disrupting chemicals may ... [https://www.endocrine.org/news-and-advocacy/news-room/endo-annual-meeting/endo-2025-press-releases/hilz-press-release]
- 6. Overview of Endocrine Disruption | US EPA [https://www.epa.gov/endocrine-disruption/overview-endocrine-disruption]
- 7. Endocrine Disruptors in Cosmetic Products and the ... [https://www.mdpi.com/2079-9284/10/6/160]
- 8. Review Endocrine disrupting chemicals exposure and health [https://www.sciencedirect.com/science/article/pii/S0147651325009194]
- 9. Endocrine-Disrupting Chemicals and Male Infertility [https://pmc.ncbi.nlm.nih.gov/articles/PMC12565704/]
- 10. Associations Between Endocrine-Disrupting Chemical ... [https://www.mdpi.com/2075-1729/15/7/993]
- 11. Endocrine-disrupting chemicals exposure: cardiometabolic ... [https://cardiab.biomedcentral.com/articles/10.1186/s12933-025-02938-8]
- 12. Association between exposure to airborne endocrine ... [https://www.sciencedirect.com/science/article/pii/S0269749125002039]
- 13. Development and validation of a survey on reproductive ... [https://www.frontiersin.org/journals/reproductive-health/articles/10.3389/frph.2025.1519896/full]
- 14. The impact, mechanisms and prevention strategies of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12520972/]
- 15. Potential effects of endocrine-disrupting chemicals on ... [https://www.sciencedirect.com/science/article/pii/S0147651325010462]
- 16. Inventory of possible endocrine disrupting chemicals used ... [https://pubmed.ncbi.nlm.nih.gov/40931777/]
- 17. What do people need to know about endocrine disrupting ... [https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-025-25561-4]
- 18. Associations Between Endocrine-Disrupting Chemical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12299029/]
- 19. Plasticizers: Types, Uses, Classification, Selection & ... [https://www.specialchem.com/polymer-additives/guide/plasticizers]
- 20. Global Environmental and Toxicological Data of Emerging ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11566117/]
- 21. The Rise of non-phthalate plasticizers: Serious risks to ... [https://www.sciencedirect.com/science/article/abs/pii/S2213343725006724]
- 22. Comparison of Heavy Metal Pollution, Health Risk ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12300138/]
- 23. Assessment of Carcinogenic and non-carcinogenic risk ... [https://www.nature.com/articles/s41598-024-81109-3]
- 24. Persistent Organic Pollutants: A Global Issue, A ... [https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response]
- 25. Persistent Organic Pollutants: A Global Health Threat [https://www.actenviro.com/persistent-organic-pollutants/]
- 26. Endocrine Disruptors and Their Impact on Quality of Life - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12066167/]
- 27. The impact, mechanisms and prevention strategies of ... [https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2025.1573526/full]
- 28. The impact, mechanisms and prevention strategies of ... [https://pubmed.ncbi.nlm.nih.gov/41103656/]
- 29. The potential endocrine-disrupting of fluorinated pesticides ... [https://www.sciencedirect.com/science/article/pii/S0147651324016919]
- 30. Interference with systemic negative feedback as a potential ... [https://pubmed.ncbi.nlm.nih.gov/40317127/]
- 31. The Role of Endocrine Disrupting Chemicals in ... [https://pubmed.ncbi.nlm.nih.gov/40820178/]
- 32. Phthalates and epigenetics: An emerging public health concern [https://pmc.ncbi.nlm.nih.gov/articles/PMC12639268/]
- 33. Unveiling the mediating role of oxidative stress in ... [https://www.sciencedirect.com/science/article/pii/S2773049225000352]
- 34. Decoding Environmental Exposures' Lifelong Impacts [https://magazine.publichealth.jhu.edu/2025/decoding-environmental-exposures-lifelong-impacts]
- 35. Prenatal Dietary Exposure to Endocrine Disruptors and Its ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12568251/]
- 36. Understanding the role of endocrine disrupting chemicals ... [https://www.sciencedirect.com/science/article/pii/S0147651325007377]
- 37. Plastics, EDCs & Health: Authoritative Guide [https://www.endocrine.org/topics/edc/plastics-edcs-and-health]
- 38. Prenatal exposures to mixtures of endocrine disrupting ... [https://www.nature.com/articles/s41598-021-89846-5]
- 39. Exposure to endocrine disrupting chemicals from beverage ... [https://www.sciencedirect.com/science/article/pii/S0304389423025980]
- 40. Determinants of Exposure to Endocrine Disruptors ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9884094/]
- 41. Origin, dietary exposure, and toxicity of endocrine-disrupting ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10395372/]
- 42. Endocrine-Disrupting Chemicals (EDCs) [https://www.endocrine.org/patient-engagement/endocrine-library/edcs]
- 43. Early Life Exposure to Endocrine Disrupting Chemicals and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5322271/]
- 44. Exposure Assessment Tools - NCI [https://dceg.cancer.gov/tools/exposure-assessment-tools]
- 45. Study of Use of Products and Exposure-Related Behaviors ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2940867/]
- 46. Consumer behaviour survey for assessing exposure from ... [https://www.nature.com/articles/s41370-018-0040-2]
- 47. Internal and external microplastic exposure in young adults [https://www.sciencedirect.com/science/article/pii/S0013935124021571]
- 48. A pilot study on the cutaneous effects of ethanol in ... [https://www.nature.com/articles/s41598-025-18487-9]
- 49. Population‐Based Cosmetics Exposure Parameters [https://pmc.ncbi.nlm.nih.gov/articles/PMC12481470/]
- 50. Non-invasive matrices in human biomonitoring: A review [https://www.sciencedirect.com/science/article/abs/pii/S0160412008001992]
- 51. The Screening and Correlation of Trace Elements in ... [https://www.mdpi.com/2305-6304/13/6/431]
- 52. Advances in biomonitoring technologies for women's health [https://www.nature.com/articles/s41467-025-63501-3]
- 53. Endocrine-Disrupting Chemicals and Male Infertility [https://www.mdpi.com/2039-4713/15/5/165]
- 54. Top Electronic Data Capture (EDC) Systems for Clinical Trials in 2025 – Full Comparison Guide [https://ccrps.org/clinical-research-blog/directory-of-electronic-data-capture-edc-systems-for-clinical-trials]
- 55. Electronic Data Capture (EDC) Systems Explained [https://ccrps.org/clinical-research-blog/electronic-data-capture-edc-systems]
- 56. Online Electronic Data Capture and Research Data ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6170092/]
- 57. What Is an EDC Platform For Clinical Trials? [https://www.labkey.com/edc-platform-clinical-trials/]
- 58. The Fundamentals of Clinical Trial Data Integration [https://www.quanticate.com/blog/the-fundamentals-of-clinical-trial-data-integration]
- 59. Rave Electronic Data Capture (EDC) System | Medidata Solutions [https://www.medidata.com/en/clinical-trial-products/clinical-data-management/edc-systems/]
- 60. Development and validation of a survey on reproductive ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11949970/]
- 61. Inside 2025's Smart Medical Wearables: Top Devices [https://thebusinesslegacy.com/smart-wearables-2025/]
- 62. End-to-end design of wearable sensors [https://www.nature.com/articles/s41578-022-00460-x]
- 63. Exploring Wearable Sensors for Therapeutic Drug Monitoring ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10377150/]
- 64. How to Validate Your Electronic Data Capture (EDC) System [https://preludeedc.com/resource/how-to-validate-your-edc/]
- 65. Do You Need to Validate Your CTMS After Every Update? ... [https://realtime-eclinical.com/2025/07/10/do-you-need-to-validate-your-ctms-after-every-update-heres-what-research-sites-should-know/]
- 66. 2025 Clinical Data Trend Report [https://www.veeva.com/2025-clinical-data-trend-report/]
- 67. Assessment of mechanisms driving non-linear dose ... [https://www.sciencedirect.com/science/article/pii/S1383574214000684]
- 68. Assessment of mechanisms driving non-linear dose ... [https://pubmed.ncbi.nlm.nih.gov/25795120/]
- 69. Why dose-response relationships are often non-linear and ... [https://pubmed.ncbi.nlm.nih.gov/8260836/]
- 70. Endocrine Disrupting Chemicals' Effects on Reproductive ... [https://beyondpesticides.org/dailynewsblog/2025/07/endocrine-disruptors-effects-on-reproductive-health/]
- 71. Impact of environmental endocrine disruptors on ... [https://link.springer.com/article/10.1007/s42452-025-07837-x]
- 72. Nonlinear Mixed-Effects Modeling of Population ... [https://www.mathworks.com/company/technical-articles/nonlinear-mixed-effects-modeling-of-population-pharmacokinetics-data.html]
- 73. Efficient Pharmacokinetic Modeling Workflow With the ... [https://pubmed.ncbi.nlm.nih.gov/32036625/]
- 74. Human Health Risk Assessment [https://www.epa.gov/risk/human-health-risk-assessment]
- 75. Endocrine-disrupting chemicals exposure: cardiometabolic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12487381/]
- 76. Conducting a Human Health Risk Assessment [https://www.epa.gov/risk/conducting-human-health-risk-assessment]
- 77. Exposure and potential risks of thirteen endocrine ... [https://www.sciencedirect.com/science/article/pii/S0160412024006184]
- 78. Monitoring [https://www.eu-parc.eu/thematic-areas/risk-assessments/monitoring]
- 79. New approach methodologies in human health risk ... [https://www.sciencedirect.com/science/article/pii/S0160412025000303]
- 80. Intergenerational and transgenerational effects of ... [https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2025.1571689/full]
- 81. Effects of Endocrine-Disrupting Chemicals in the Brain [https://www.mdpi.com/2039-4713/15/5/162]
- 82. Mini-Review: Epigenetic mechanisms that promote transgenerational ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9942829/]
- 83. Tech Science Press - Publisher of Open Access Journals [https://www.techscience.com/biocell/special_detail/endocrine_disruptors_cellular_mechanisms]
- 84. Endocrine Disrupting Chemicals and Gender Dysphoria. [https://www.linkedin.com/pulse/endocrine-disrupting-chemicals-gender-dysphoria-mark-gordon]
- 85. Integrative causal inference illuminates gene-environment ... [https://www.sciencedirect.com/science/article/pii/S0147651325010243]
- 86. Deciding What to Add in a Cumulative Risk Assessment [https://pmc.ncbi.nlm.nih.gov/articles/PMC9262140/]
- 87. Mixture math: Deciding what to add in a cumulative risk ... [https://www.sciencedirect.com/science/article/abs/pii/S2468202022000419]
- 88. Origin, dietary exposure, and toxicity of endocrine ... [https://www.sciencedirect.com/science/article/pii/S2405844023053483]
- 89. Reducing Exposures to Endocrine Disruptors (REED) study, a ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11587698/]
- 90. What You Can Do About EDCs [https://www.endocrine.org/topics/edc/what-you-can-do]
- 91. The Quality Issue - January 2025 - TRI - TriTrials [https://www.tritrials.com/the-quality-issue-january-2025/]
- 92. What is Risk-Based Monitoring (RBM)? [https://www.transceleratebiopharmainc.com/rbminteractiveguide/what-is-risk-based-monitoring-rbm/introduction/]
- 93. Data Quality Measurement 2025 Trends and Proven ... [https://www.bitechnology.com/data-quality-measurement-2025-trends-and-proven-improvement-methodologies/]
- 94. How to Improve Data Quality: A Strategic Guide for 2025 [https://www.ewsolutions.com/how-to-improve-data-quality/]
- 95. The Best Electronic Data Capture (EDC) Systems in 2025 [https://swissdidata.com/blog/best-edc-systems]
- 96. News from CRIS: Everyday Toxicology - Weight of Evidence [https://iit.msu.edu/news/2025-5-19-CRIS-everyday-toxicology-weight-evidence.html]
- 97. The intracellular free concentration of endocrine disrupting ... [https://pubmed.ncbi.nlm.nih.gov/40562181/]
- 98. Refinement of a workflow for human relevance assessment ... [https://www.frontiersin.org/journals/toxicology/articles/10.3389/ftox.2025.1616817/full]
- 99. Weight of evidence evaluation of the metabolism disrupting ... [https://www.sciencedirect.com/science/article/pii/S0041008X24001935]
- 100. March 2025 – EU Omnibus package, US EPA reconsiders ... [https://www.spglobal.com/sustainable1/en/insights/regulatory-tracker/esg-regulatory-tracker-march-2025]
- 101. U.S.–European Sustainability Policy Divergence Grows [https://natlawreview.com/article/transatlantic-divide-widens-us-vs-eu-2025-sustainability-policy]
- 102. How US sustainability regulations compare to EU ... [https://instituteofsustainabilitystudies.com/insights/lexicon/how-us-sustainability-regulations-compare-to-eu-standards-in-2025/]
- 103. Association between environmental endocrine disruptors and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12637241/]
- 104. Behavioral Intervention Technologies: Evidence review ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3719158/]
- 105. Behavioral Intervention Technologies: Evidence review ... [https://www.sciencedirect.com/science/article/pii/S0163834313000698]
- 106. NIH Stage Model for Behavioral Intervention Development [https://www.nia.nih.gov/research/dbsr/nih-stage-model-behavioral-intervention-development]
- 107. Exposure Assessment Tools by Routes - Dermal | US EPA [https://www.epa.gov/expobox/exposure-assessment-tools-routes-dermal]
- 108. Exposure Assessment Tools by Routes - Ingestion | US EPA [https://www.epa.gov/expobox/exposure-assessment-tools-routes-ingestion]
- 109. Behavioral Counseling Interventions: An Evidence-based ... [https://www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/behavioral-counseling-interventions-evidence-based-approach]
- 110. Performance of transdermal therapeutic systems: Effects ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3465120/]
- 111. Volatile Organic Compounds in Air: Sources, Distribution ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6748254/]
- 112. The relation between inflammatory biomarkers and drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11577668/]
- 113. Pharmacokinetic models of dermal absorption [https://www.sciencedirect.com/science/article/pii/S0022354916308735]
- 114. Use of Biomarkers in Drug Development for Regulatory ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12489174/]
- 115. Dermal Absorption [https://www.labcorp.com/industries/crop-protection/environmental-safety/environmental-fate-metabolism/dermal-absorption]
- 116. What to expect in the clinical trials industry in 2025 [https://www.viedoc.com/blog/what-to-expect-clinical-trials-2025]